chair conformation energy diagram These structural and energetic relationships are summarized in the conformational energy diagram for cyclohexane below. With the chair on the left of the figure the nose of the cyclohexane chair goes down and the tail of the cyclohexane goes up to make the new chair cyclohexane conformer.
Chair Conformation Energy Diagram, The energy barriers between the chair boat and twist conformations of cyclohexane are low enough Fig6 to make separation of the. With a ring flip all hydrogens that were originally axial become equatorial and all hydrogens that were equatorial become axial. The energy required for this ring-flipping process is shown in Figure 615.
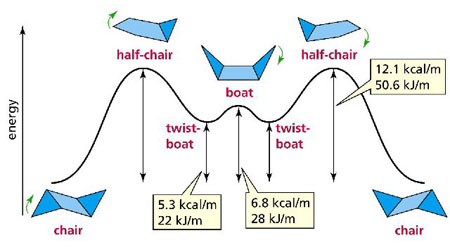 Stereochemistry Of Cyclohexane Chemistry Revision Site From ueache.weebly.com
Stereochemistry Of Cyclohexane Chemistry Revision Site From ueache.weebly.com
Another Article :
14 Which of the statements below correctly describes the chair conformations of trans-13-. The energy barriers between the chair boat and twist conformations of cyclohexane are low enough Fig6 to make separation of the. Rather the carbon skele-ton is puckered. The free energy ΔG of the equilibrium process going from one conformer to the other ie from A to B as shown in step 4 or 5 can be calculated from the equation below where the. Notice the position of this conformation on the energy diagram of Fig.
There are two puckered conformations of cyclopentane the envelope IHIPOE ACUHUB and the half-chair LISLOO ABIKUR.
For most classes all you will need to know how to do is use equatorial preference to predict the most stable chair conformation. This conformation of cyclohexane is called the chair conformation be-cause of its resemblance to a lawn chair. The most stable conformation of cyclohexane is shown in Fig. The free energy ΔG of the equilibrium process going from one conformer to the other ie from A to B as shown in step 4 or 5 can be calculated from the equation below where the. With the chair on the left of the figure the nose of the cyclohexane chair goes down and the tail of the cyclohexane goes up to make the new chair cyclohexane conformer.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
Energy Profile Of Cyclohexene Conformations Chemistry Stack Exchange For each chair conformer add the energy of all the groups on axial position. This is a multistep process so here Im going to walk you through it from scratch. However sometimes you will be required to use energetics to calculate the exact percentages of each chair in solution. The energy required for this ring-flipping process is shown in Figure 615. Newman Projection Energy Diagrams. This is the half-chair conformation of cyclo-hexane and it is the transition state for the interconversion of the chair and twist-boat confor-mations.

Ring Flipping In Cyclohexane In A Different Light Henry Rzepa S Blog For most classes all you will need to know how to do is use equatorial preference to predict the most stable chair conformation. The Chair Conformation - a closer look Since the chair conformation has the lowest potential energy it is the most relevant to the conformation of cyclohexane. The energy barriers between the chair boat and twist conformations of cyclohexane are low enough Fig6 to make separation of the. With the chair on the left of the figure the nose of the cyclohexane chair goes down and the tail of the cyclohexane goes up to make the new chair cyclohexane conformer. Energy Diagram Of The Cyclohexane Chair Flip. These structural and energetic relationships are summarized in the conformational energy diagram for cyclohexane below.
 Source: research.cm.utexas.edu
Source: research.cm.utexas.edu
Cyclohexane Conformational Analysis The free energy ΔG of the equilibrium process going from one conformer to the other ie from A to B as shown in step 4 or 5 can be calculated from the equation below where the. Draw a qualitative energy diagram for CH 3CH 2CH 2CHCH 3 2 relative to the bond between the two CH2 carbons. This is the half-chair conformation of cyclo-hexane and it is the transition state for the interconversion of the chair and twist-boat confor-mations. With a ring flip all hydrogens that were originally axial become equatorial and all hydrogens that were equatorial become axial. Notice the position of this conformation on the energy diagram of Fig. The chair conformation is estimated to be lower in energy than the twist conformation by approximately 23 kJ mol-1.
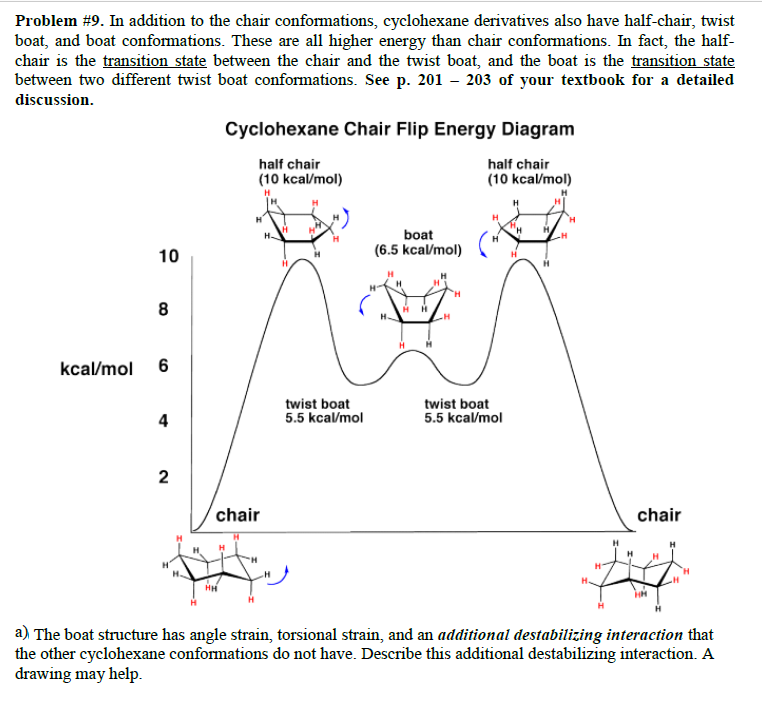 Source: chegg.com
Source: chegg.com
Solved Problem 9 In Addition To The Chair Conformations Chegg Com For most classes all you will need to know how to do is use equatorial preference to predict the most stable chair conformation. Rather the carbon skele-ton is puckered. 75 Give two reasons why this conformation is less stable than the chair or twist-boat conformation. For most classes all you will need to know how to do is use equatorial preference to predict the most stable chair conformation. If you have not already done so you should. In the last post we showed a video of a cyclohexane ring flip turning a cyclohexane chair conformation into a boat and then into the opposite chair.
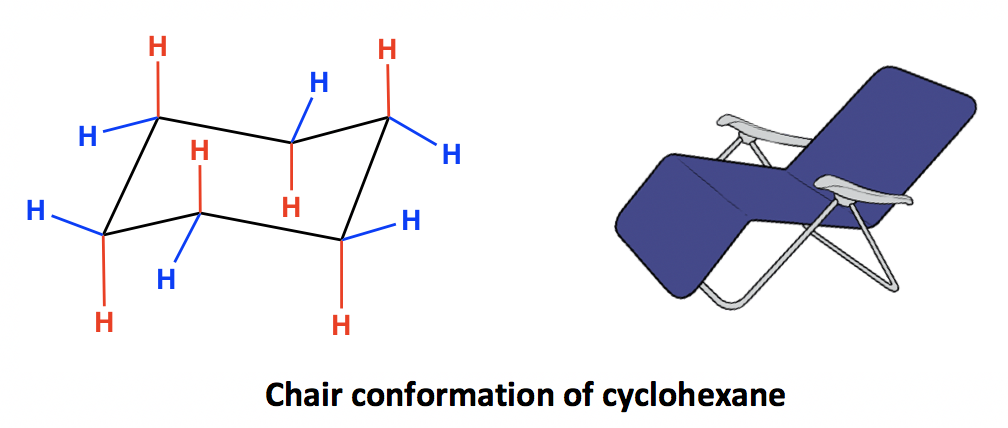 Source: kpu.pressbooks.pub
Source: kpu.pressbooks.pub
4 3 Conformation Analysis Of Cyclohexane Organic Chemistry And now the stabilities. Here we have the butane molecule and this is carbon one carbon two carbon three and finally carbon four and if we stare down the carbon two three bond so here Im rotating the molecules so we stare down the carbon two three bonds this is a staggered conformation of butane and if we rotate if you rotate the front carbon to keep the back carbon stationary and rotate sixty degrees and were going to get an Eclipse conformation. For most classes all you will need to know how to do is use equatorial preference to predict the most stable chair conformation. The Newman projections are drawn below using iPr as an abbreviation for the isopropyl CHCH 3 2 group. These structural and energetic relationships are summarized in the conformational energy diagram for cyclohexane below. The minimum energy conformation adopted is therefore a balance of the two opposing types of strain.
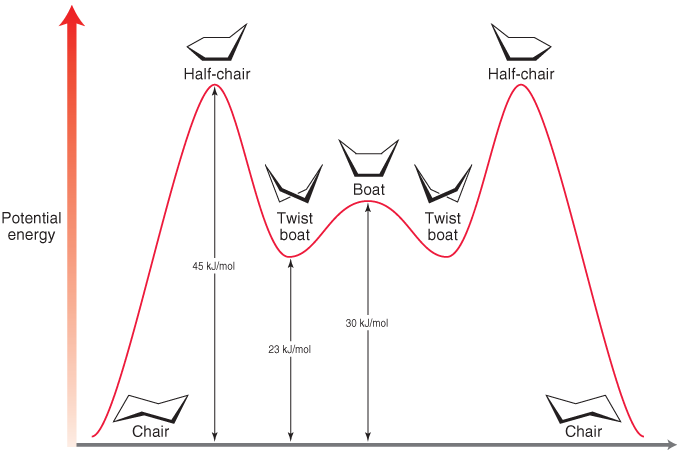 Source: chegg.com
Source: chegg.com
Solved The Chair Conformation Is The Most Favorable Chegg Com To equatorial hydrogens in the right chair. Draw the second chair conformation ring-flip-check this post if not sure. The most stable conformation of cyclohexane is shown in Fig. The energy required for this ring-flipping process is shown in Figure 615. D The higher energy chair conformation contains two axial methyl groups. CYCLOHEXANE CONFORMATIONAL ENERGY DIAGRAM.
 Source: chemgapedia.de
Source: chemgapedia.de
Cycloalkanes Structural Variety Chemgapedia However all up groups remain up and. Draw the second chair conformation ring-flip-check this post if not sure. Energy Diagram Of The Cyclohexane Chair Flip. For most classes all you will need to know how to do is use equatorial preference to predict the most stable chair conformation. The highest barrier called the half-chair conformation is about 105 kcalmol 44 kJmol higher in energy than the chair conformation. To equatorial hydrogens in the right chair.
 Source: ueache.weebly.com
Source: ueache.weebly.com
Stereochemistry Of Cyclohexane Chemistry Revision Site In the last post we showed a video of a cyclohexane ring flip turning a cyclohexane chair conformation into a boat and then into the opposite chair. D The higher energy chair conformation contains two axial methyl groups. And now the stabilities. However sometimes you will be required to use energetics to calculate the exact percentages of each chair in solution. Rather the carbon skele-ton is puckered. In this con-formation of cyclohexane the carbons do not lie in a single plane.

What Is The Order Of Stability Of The Conformers Of Cyclohexane Why Quora However sometimes you will be required to use energetics to calculate the exact percentages of each chair in solution. However all up groups remain up and. The following guidelines can be used to determine the percent distribution of two chair conformers using the Gibbs free energy equation and some simple algebra. Rather the carbon skele-ton is puckered. C The lower energy chair conformation contains one axial methyl group and one equatorial methyl group. Newman Projection Energy Diagrams.
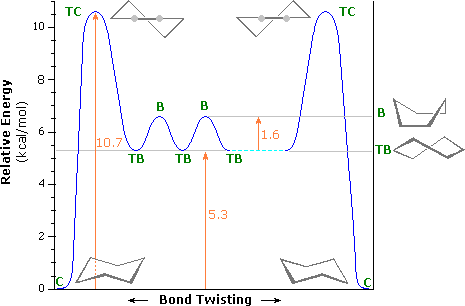 Source: chem.libretexts.org
Source: chem.libretexts.org
3 9 Some Cycloalkanes Have Angle Strain Chemistry Libretexts With the chair on the left of the figure the nose of the cyclohexane chair goes down and the tail of the cyclohexane goes up to make the new chair cyclohexane conformer. H iPr H CH3 H H H iPr. And now the stabilities. 14 Which of the statements below correctly describes the chair conformations of trans-13-. Rather the carbon skele-ton is puckered. The chair conformation is estimated to be lower in energy than the twist conformation by approximately 23 kJ mol-1.
 Source: shefalitayal.com
Source: shefalitayal.com
Cyclohexane Conformer The Cyclohexane Chair Flip Part B Cyclohexane Exists In Different Conformers Cyclohexane Stereochemistry The energy required for this ring-flipping process is shown in Figure 615. However all up groups remain up and. The most stable conformation of cyclohexane is shown in Fig. This is a multistep process so here Im going to walk you through it from scratch. To equatorial hydrogens in the right chair. In the last post we showed a video of a cyclohexane ring flip turning a cyclohexane chair conformation into a boat and then into the opposite chair.

Answer In Organic Chemistry For Rakesh Kumar 104929 Draw the second chair conformation ring-flip-check this post if not sure. However sometimes you will be required to use energetics to calculate the exact percentages of each chair in solution. The key observation we made here was that a chair flip converts all axial groups into equatorial groups and all equatorial groups into axial groups. Newman Projection Energy Diagrams. 14 Which of the statements below correctly describes the chair conformations of trans-13-. The first stage involves the transition from an equilibrium half-chair conformation to a twist-boat conformation with a significant increase in energy.
 Source: twitter.com
Source: twitter.com
Hassan Rasouli On Twitter Energy Diagram Cyclohexane Conformations Stereochemistry The second stage may be described as a very easy transformation of one twist-boat conformation to another via boat almost without any changes in energy. This is a multistep process so here Im going to walk you through it from scratch. The minimum energy conformation adopted is therefore a balance of the two opposing types of strain. If you have not already done so you should. However since this conformation is 55 kcalmol higher in energy than the chair conformation it is not a highly populated stateAgain most of the strain energy results from bonds which are eclipsed or partially eclipsed ie from torsional strain. 75 Give two reasons why this conformation is less stable than the chair or twist-boat conformation.
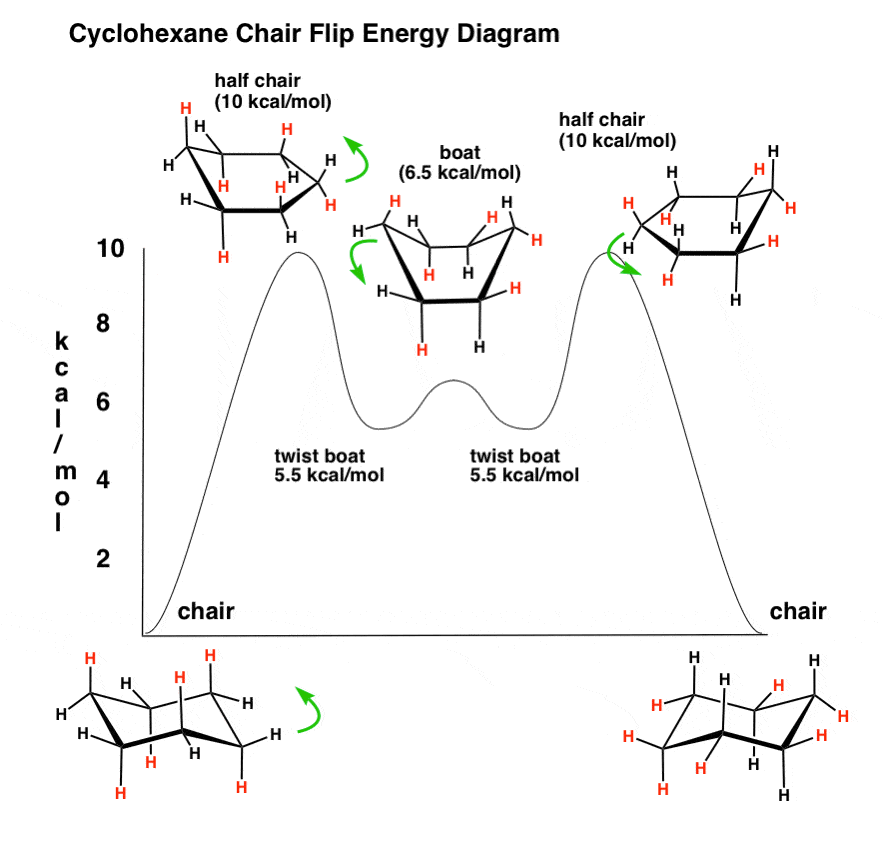 Source: brainstudy.info
Source: brainstudy.info
Why Is The Cyclohexane Ring Free Of Strain In the last post we showed a video of a cyclohexane ring flip turning a cyclohexane chair conformation into a boat and then into the opposite chair. The highest barrier called the half-chair conformation is about 105 kcalmol 44 kJmol higher in energy than the chair conformation. The key observation we made here was that a chair flip converts all axial groups into equatorial groups and all equatorial groups into axial groups. This is the half-chair conformation of cyclo-hexane and it is the transition state for the interconversion of the chair and twist-boat confor-mations. With the chair on the left of the figure the nose of the cyclohexane chair goes down and the tail of the cyclohexane goes up to make the new chair cyclohexane conformer. The chair conformation is estimated to be lower in energy than the twist conformation by approximately 23 kJ mol-1.

Ring Flipping In Cyclohexane In A Different Light Henry Rzepa S Blog Again the 20 kcalmol 837 kJmol of energy that is available at room temperature provides. Draw the second chair conformation ring-flip-check this post if not sure. To equatorial hydrogens in the right chair. In the last post we showed a video of a cyclohexane ring flip turning a cyclohexane chair conformation into a boat and then into the opposite chair. For each chair conformer add the energy of all the groups on axial position. The second stage may be described as a very easy transformation of one twist-boat conformation to another via boat almost without any changes in energy.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
Solvation Effect On Cyclohexane Chemistry Stack Exchange The second stage may be described as a very easy transformation of one twist-boat conformation to another via boat almost without any changes in energy. Notice the position of this conformation on the energy diagram of Fig. The most stable conformation of cyclohexane is shown in Fig. Again the 20 kcalmol 837 kJmol of energy that is available at room temperature provides. If you have not already done so you should. 75 Give two reasons why this conformation is less stable than the chair or twist-boat conformation.
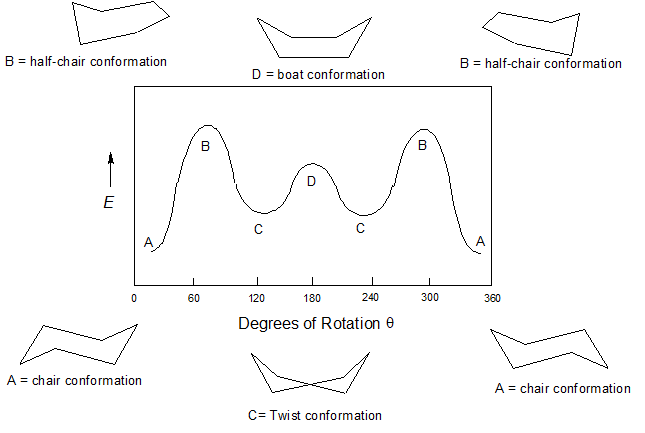 Source: chem.libretexts.org
Source: chem.libretexts.org
4 7 Cyclohexane Conformations Chemistry Libretexts Newman Projection Energy Diagrams. Least stable chair Most stable chair H3C CH3G Ð27 kcalmol d In the box below explain using appropriate diagrams if you wish why the difference in energy between the two chair conformations of trans-12-dimethylcyclohexane is 09 kcalmol LESS than the. The chair conformation is estimated to be lower in energy than the twist conformation by approximately 23 kJ mol-1. With the chair on the left of the figure the nose of the cyclohexane chair goes down and the tail of the cyclohexane goes up to make the new chair cyclohexane conformer. 14 Which of the statements below correctly describes the chair conformations of trans-13-. For each chair conformer add the energy of all the groups on axial position.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Cyclohexane Chair Conformation Stability Which One Is Lower Energy Put S for staggered by any staggered conformation and E for eclipsed by an eclipsed conformation. H iPr H CH3 H H H iPr. And now the stabilities. Notice the position of this conformation on the energy diagram of Fig. 75 Give two reasons why this conformation is less stable than the chair or twist-boat conformation. Energy Diagram Of The Cyclohexane Chair Flip.










