Chair conformation calculator Related to this Question. However with so much strain on the bonds it prefers to adopt a chair conformation.
Chair Conformation Calculator, Chair Conformation of Cyclohexane A chair conformation is the. Least stable chair Most stable chair 1 1 1 Ð18 kcalmolG b Chlorocyclohexane also exists in two different chair conformations one of which is 06 kcalmol more stable than the other ie the A value for the chloro group is 06. Six hydrogen centers are poised in axial positions roughly parallel with the C 3 axis and six hydrogen atoms are located near the equator.
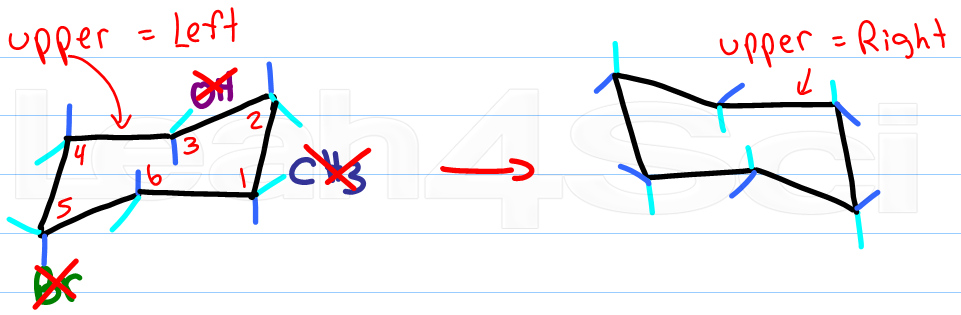 Drawing Chair Conformations And Ring Flips For Cyclohexane In Organic Chemistry From leah4sci.com
Drawing Chair Conformations And Ring Flips For Cyclohexane In Organic Chemistry From leah4sci.com
Another Article :
Draw both chair conformation ring-flip and use the table to calculate the relative energy cost associated with each group in the axial position to determine the more stable chair conformation of each of the following compounds. Identify the carbon number for the first substituent if its wedged add it to the up position. Chair boat and twist-boat. Works an example of translating a trisubstituted cyclohexane perspective d. Number the carbons in your cyclohexane and in your chair.
01 to 02 boat form ie.
From a cyclohexyl structure to the chair conformation remember the relationship of axial and equitorial groups and dont mix this up with cis and trans. H H HH H H H H H HH H 4 5 3 1 26 H. Lets get some practice drawing it chair confirmations one way to do it is to start by drawing two parallel lines that are offset from each others let me go ahead and show you what I mean so heres heres one line and then here is another line theyre parallel to each other but theyre offset a little bit next were going to draw two horizontal dotted lines so the top horizontal dotted line is. Chair boat and twist-boat. In the left-hand cyclohexane chair carbon atoms 13 and 5 are above an imaginary horizontal plane and carbon.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Cyclohexane Chair Conformation Stability Which One Is Lower Energy As we can see from the above calculations as the temperature rises the percentage contribution by equatorial conformation decreases while that by axial conformation increases. However sometimes you will be required to use energetics to calculate the exact percentages of each chair in solutionThis is a multistep process so here Im going to walk you through it from scratch. The proper conformation of cyclohexane is not a hexane. However with so much strain on the bonds it prefers to adopt a chair conformation. H H HH H H H H H HH H 4 5 3 1 26 H. The final piece of these conversions is often to draw a complete chair conformation of the pyranose.
 Source: leah4sci.com
Source: leah4sci.com
Drawing Chair Conformations And Ring Flips For Cyclohexane In Organic Chemistry Six hydrogen centers are poised in axial positions roughly parallel with the C 3 axis and six hydrogen atoms are located near the equator. 1 or 2 molecules per thousand. Practice will make perfect. 1 1 4 4 In the illustration above the two chair conformations are in equilibrium. 01 to 02 boat form ie. The proper conformation of cyclohexane is not a hexane.
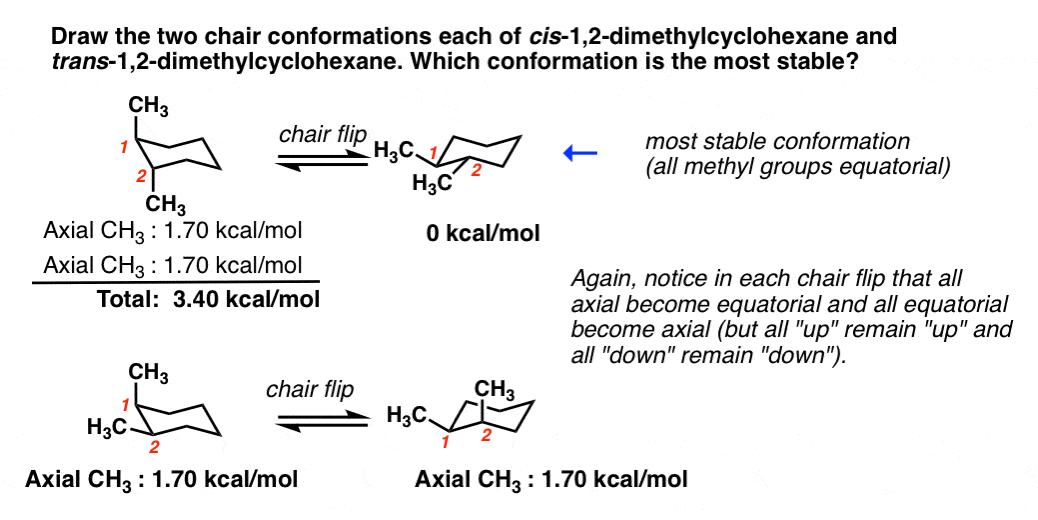 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Cyclohexane Chair Conformation Stability Which One Is Lower Energy It has 2971kJmol more energy than chair conformation. The symmetry is D3d. Clockwise or counterclockwise doesnt matter as long as you use the same direction for both molecules. Finally add an upward-pointing V tip to the other end this is the nose of the chair. From a cyclohexyl structure to the chair conformation remember the relationship of axial and equitorial groups and dont mix this up with cis and trans. Converting Haworth to Chair.
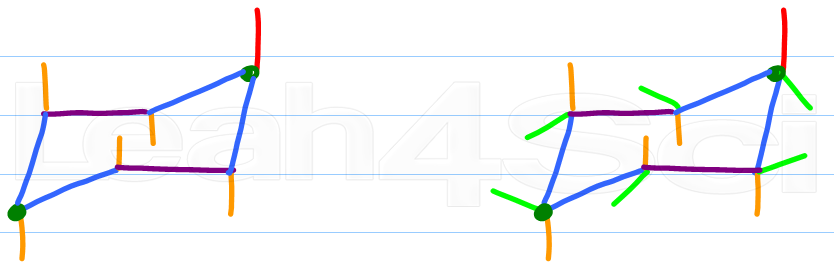 Source: leah4sci.com
Source: leah4sci.com
Drawing Chair Conformations And Ring Flips For Cyclohexane In Organic Chemistry 2 Calculate percent contribution by each conformation of cis-1-tButyl-2-methylcyclohexane at 25C. At 25 C 9999 of all molecules in a cyclohexane solution adopt this conformation. 1 1 4 4 In the illustration above the two chair conformations are in equilibrium. 01 to 02 boat form ie. Therefore boat conformation is less stable than chair conformation. In each of the two boxes below draw in a bond to one chloro Cl group in the appropriate position.
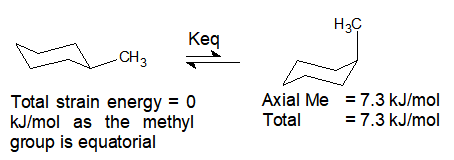 Source: organicchemistrytutorials.com
Source: organicchemistrytutorials.com
Blog 05 Calculate Percentage Of Chair Conformation In Cyclohexane In the left-hand cyclohexane chair carbon atoms 13 and 5 are above an imaginary horizontal plane and carbon. Least stable chair Most stable chair 1 1 1 Ð18 kcalmolG b Chlorocyclohexane also exists in two different chair conformations one of which is 06 kcalmol more stable than the other ie the A value for the chloro group is 06. Six hydrogen centers are poised in axial positions roughly parallel with the C 3 axis and six hydrogen atoms are located near the equator. Converting Haworth to Chair. Lets now examine how to calculate equlibrium concentrations of cyclohexane conformers. The tert-butyl group needs to be placed in the equatorial bond as it is the lowest energy or highest stability conformation.
 Source: leah4sci.com
Source: leah4sci.com
Drawing Chair Conformations And Ring Flips For Cyclohexane In Organic Chemistry The tert-butyl group needs to be placed in the equatorial bond as it is the lowest energy or highest stability conformation. Cyclohexane Chair Conformations and Ring Flips. There are two equivalent chair conformations. Six hydrogen centers are poised in axial positions roughly parallel with the C 3 axis and six hydrogen atoms are located near the equator. Cyclohexane is often depicted as a planar hexagon. Draw both chair conformation ring-flip and use the table to calculate the relative energy cost associated with each group in the axial position to determine the more stable chair conformation of each of the following compounds.
 Source: chemistrysteps.com
Source: chemistrysteps.com
Ring Flip Comparing The Stability Of Chair Conformations With Practice Problems Chemistry Steps Lets now examine how to calculate equlibrium concentrations of cyclohexane conformers. It has 2971kJmol more energy than chair conformation. The chair conformation is the most stable conformer. Cyclohexane Chair Conformations and Ring Flips. One HH 4 kJmol and two HCH 3 2 x 6 kJmol 12 kJmol. Cyclohexane is often depicted as a planar hexagon.
 Source: leah4sci.com
Source: leah4sci.com
Drawing Chair Conformations And Ring Flips For Cyclohexane In Organic Chemistry The steps involved in drawing the chair conformation of cyclohexane. The final piece of these conversions is often to draw a complete chair conformation of the pyranose. The second conformation is three pairs of eclipsing groups. Start with a blank chair conformation. As we can see from the above calculations as the temperature rises the percentage contribution by equatorial conformation decreases while that by axial conformation increases. In the left-hand cyclohexane chair carbon atoms 13 and 5 are above an imaginary horizontal plane and carbon.
 Source: clutchprep.com
Source: clutchprep.com
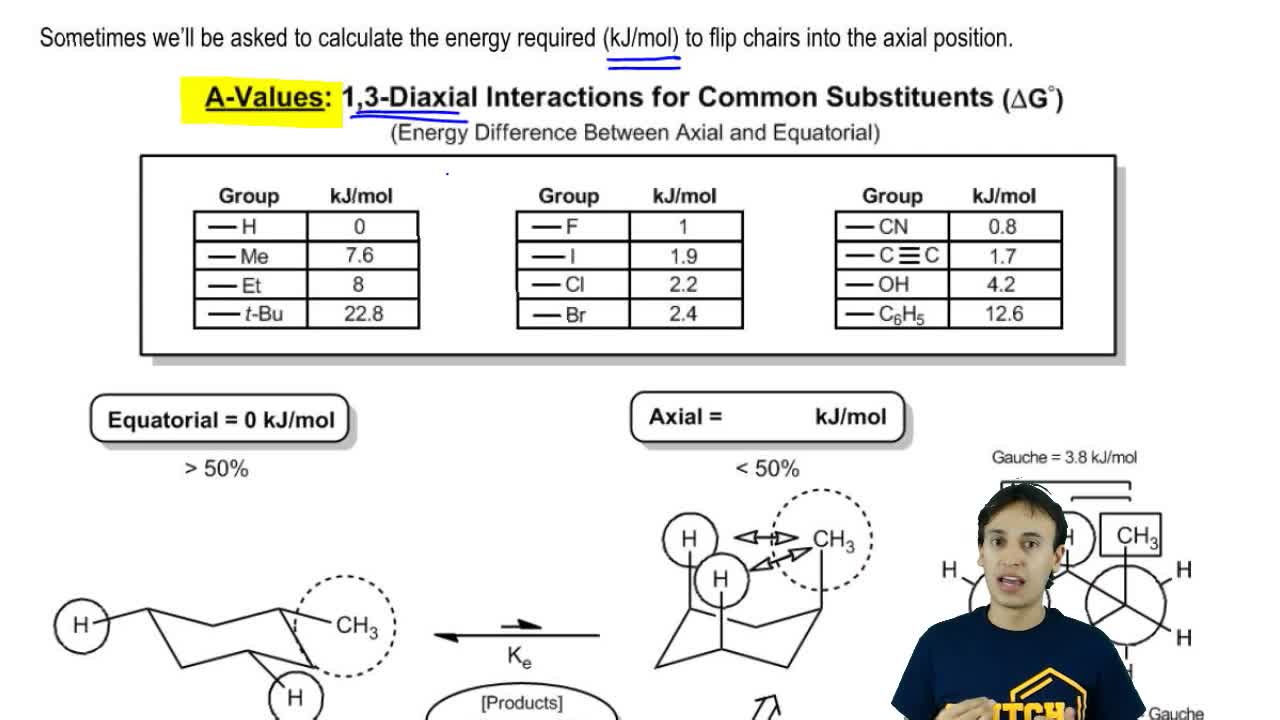
Calculating Energy Difference Between Chair Conformations Organic Chemistry Video Clutch Prep The tert-butyl group needs to be placed in the equatorial bond as it is the lowest energy or highest stability conformation. However with so much strain on the bonds it prefers to adopt a chair conformation. It has 2971kJmol more energy than chair conformation. In the left-hand cyclohexane chair carbon atoms 13 and 5 are above an imaginary horizontal plane and carbon. Number the carbons in your cyclohexane and in your chair. Converting Haworth to Chair.
 Source: clutchprep.com
Source: clutchprep.com
Calculating Energy Difference Between Chair Conformations Organic Chemistry Video Clutch Prep Chair Conformation of Cyclohexane A chair conformation is the. Next add a downward-pointing V tip to one end this is the tail of the chair. When one chair conformation flips into the other the axial and equatorial hydrogens interconvert. The steps involved in drawing the chair conformation of cyclohexane. Clockwise or counterclockwise doesnt matter as long as you use the same direction for both molecules. Practice will make perfect.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
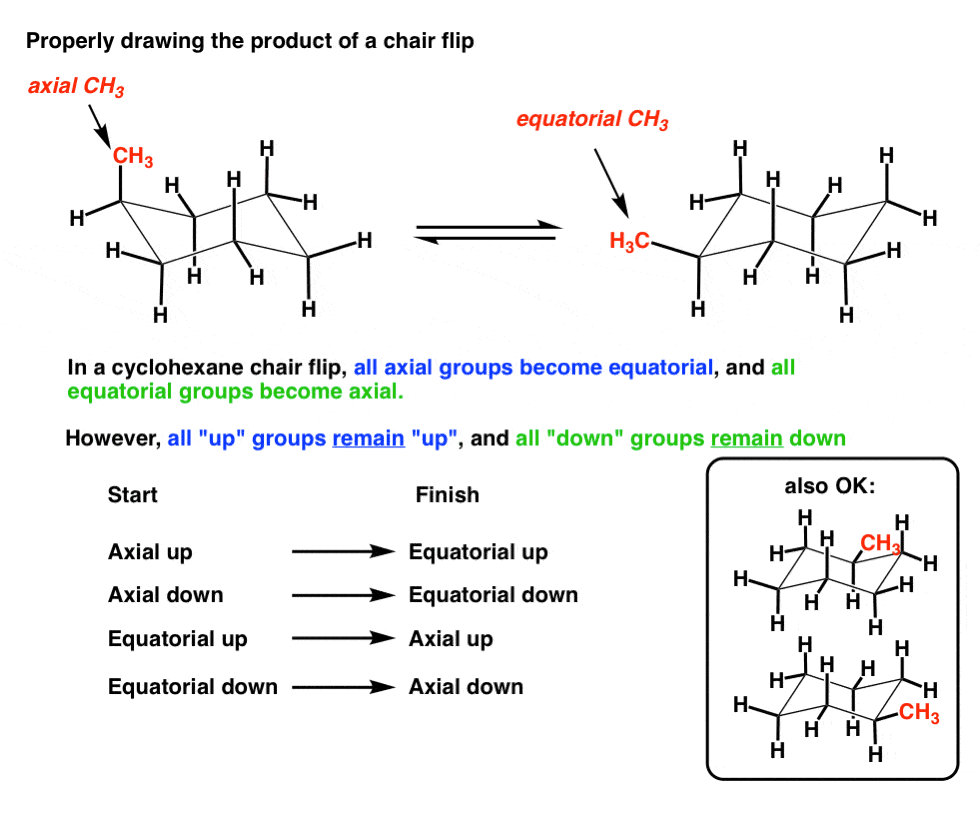
The Cyclohexane Chair Flip Master Organic Chemistry H H HH H H H H H HH H 4 5 3 1 26 H. Start with a blank chair conformation. Lets get some practice drawing it chair confirmations one way to do it is to start by drawing two parallel lines that are offset from each others let me go ahead and show you what I mean so heres heres one line and then here is another line theyre parallel to each other but theyre offset a little bit next were going to draw two horizontal dotted lines so the top horizontal dotted line is. Least stable chair Most stable chair 1 1 1 Ð18 kcalmolG b Chlorocyclohexane also exists in two different chair conformations one of which is 06 kcalmol more stable than the other ie the A value for the chloro group is 06. Chair boat and twist-boat. Lets now examine how to calculate equlibrium concentrations of cyclohexane conformers.
 Source: chemistrysteps.com
Source: chemistrysteps.com
Ring Flip Comparing The Stability Of Chair Conformations With Practice Problems Chemistry Steps The trick is to remember that just like the Haworth projections the chair conformations also have the well-defined up and down positions. Yet one conformation is preferred over the other due to the energies associated with the substituent when positioned on the chair. Next add a downward-pointing V tip to one end this is the tail of the chair. Cyclohexane is often depicted as a planar hexagon. In the left-hand cyclohexane chair carbon atoms 13 and 5 are above an imaginary horizontal plane and carbon. All carbon centers are equivalent.
 Source: clutchprep.com
Source: clutchprep.com
Calculating Energy Difference Between Chair Conformations Organic Chemistry Video Clutch Prep Least stable chair Most stable chair 1 1 1 Ð18 kcalmolG b Chlorocyclohexane also exists in two different chair conformations one of which is 06 kcalmol more stable than the other ie the A value for the chloro group is 06. However sometimes you will be required to use energetics to calculate the exact percentages of each chair in solutionThis is a multistep process so here Im going to walk you through it from scratch. In this lesson we will learn about the three conformations that cyclohexane can form. Chair boat and twist-boat. One HH 4 kJmol and two HCH 3 2 x 6 kJmol 12 kJmol. There are two equivalent chair conformations.
 Source: youtube.com
Source: youtube.com
Percent Conformers Axial Vs Equitorial Of Cyclohexanes Youtube The energy of the diaxial conformation is 4 x 09 or 2 x 18 36 kcalmol. Cyclohexane is often depicted as a planar hexagon. Related to this Question. There are two equivalent chair conformations. Practice will make perfect. Next add a downward-pointing V tip to one end this is the tail of the chair.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
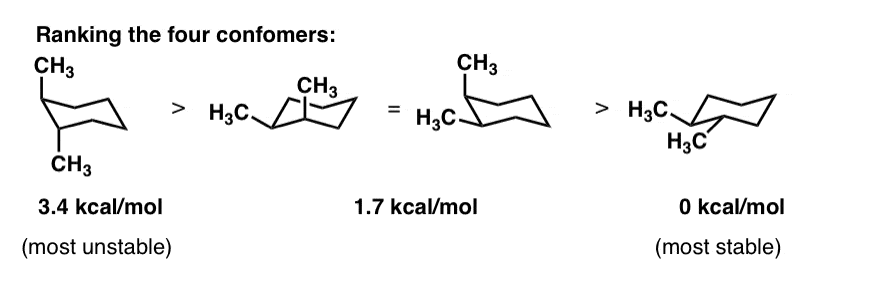
Cyclohexane Chair Conformation Stability Which One Is Lower Energy All carbon centers are equivalent. Practice will make perfect. 1 or 2 molecules per thousand. Cyclohexane is often depicted as a planar hexagon. The first conformation has one CH 3 CH 3 gauche interaction which brings 38 kJmol energy of destabilization. Chair Conformation of Cyclohexane A chair conformation is the.
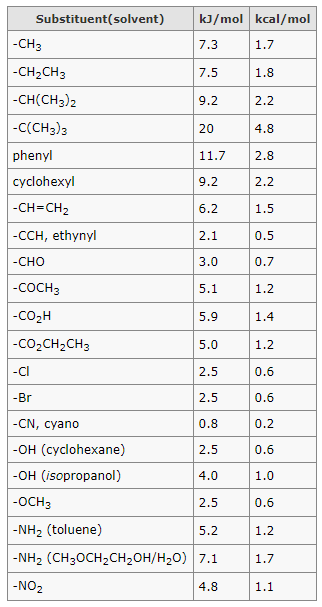 Source: organicchemistrytutorials.com
Source: organicchemistrytutorials.com
Blog 05 Calculate Percentage Of Chair Conformation In Cyclohexane Count the number of axial methyl groups or any other group for that matter that bear a 13-relationship to axial hydrogens. Chair Conformation of Cyclohexane A chair conformation is the. 2 Calculate percent contribution by each conformation of cis-1-tButyl-2-methylcyclohexane at 25C. It has 2971kJmol more energy than chair conformation. Count the number of axial methyl groups or any other group for that matter that bear a 13-relationship to axial hydrogens. Finally add an upward-pointing V tip to the other end this is the nose of the chair.
 Source: clutchprep.com
Source: clutchprep.com
Calculating Energy Difference Between Chair Conformations Organic Chemistry Video Clutch Prep The proper conformation of cyclohexane is not a hexane. However sometimes you will be required to use energetics to calculate the exact percentages of each chair in solutionThis is a multistep process so here Im going to walk you through it from scratch. The second conformation is three pairs of eclipsing groups. The tert-butyl group needs to be placed in the equatorial bond as it is the lowest energy or highest stability conformation. This tutorial series will teach you everything from understanding these structures drawing chair conformations and ranking stability based on substituents. Draw both chair conformation ring-flip and use the table to calculate the relative energy cost associated with each group in the axial position to determine the more stable chair conformation of each of the following compounds.
 Source: clutchprep.com
Source: clutchprep.com
Calculating Energy Difference Between Chair Conformations Organic Chemistry Video Clutch Prep Start with a blank chair conformation. However with so much strain on the bonds it prefers to adopt a chair conformation. Works an example of translating a trisubstituted cyclohexane perspective d. The tert-butyl group needs to be placed in the equatorial bond as it is the lowest energy or highest stability conformation. Six hydrogen centers are poised in axial positions roughly parallel with the C 3 axis and six hydrogen atoms are located near the equator. Thus the diaxial conformation is 27.
Please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save or be able to bookmark this blog page in this website. Thank you …










