Chair conformation cis trans 20 points Write both chair conformations for both the trans and cis isomers of 4-methylcyclohexanol shown in a flat-ring structure below. The azide N 3- nucleophile must attack the epoxide from behind in order to interact with the C-O σ orbital.
Chair Conformation Cis Trans, Furthermore since 14-axax-bonds are always trans opposite direction. Chair conformations Cis and Trans. Lets get some practice drawing it chair confirmations one way to do it is to start by drawing two parallel lines that are offset from each others let me go ahead and show you what I mean so heres heres one line and then here is another line theyre parallel to each other but theyre offset a little bit next were going to draw two horizontal dotted lines so the top horizontal dotted line is going to be on level with that top.
 Cis And Trans Substituent Relationships Organic Chemistry I Youtube From youtube.com
Cis And Trans Substituent Relationships Organic Chemistry I Youtube From youtube.com
Another Article :
Compounds with 14-axax-substitutions are always trans. 20 points Write both chair conformations for both the trans and cis isomers of 4-methylcyclohexanol shown in a flat-ring structure below. Furthermore since 14-axax-bonds are always trans opposite direction. Different spatial arrangements of atoms that result from rotations about single σ bonds. Trans-decalin is a kind of rigid system and not like cyclohexane both the rings of the trans-decalin cannot be flip from one form of a chair into another form.
Which is more stable cis- or trans- isomers.
A lot of students say Oh but theyre both equatorial so that means that they should be cis. The dihedral angle between two hydrogen atoms on adjacent carbon atoms on the same side of the ring is 55º. A particularly important case comes up with. Chair concordances can present the challenge in organic chemistry. Ok so I am going to start out with discussing cis-decalin and trans-decalin which are diastereomers a type of stereoisomers.
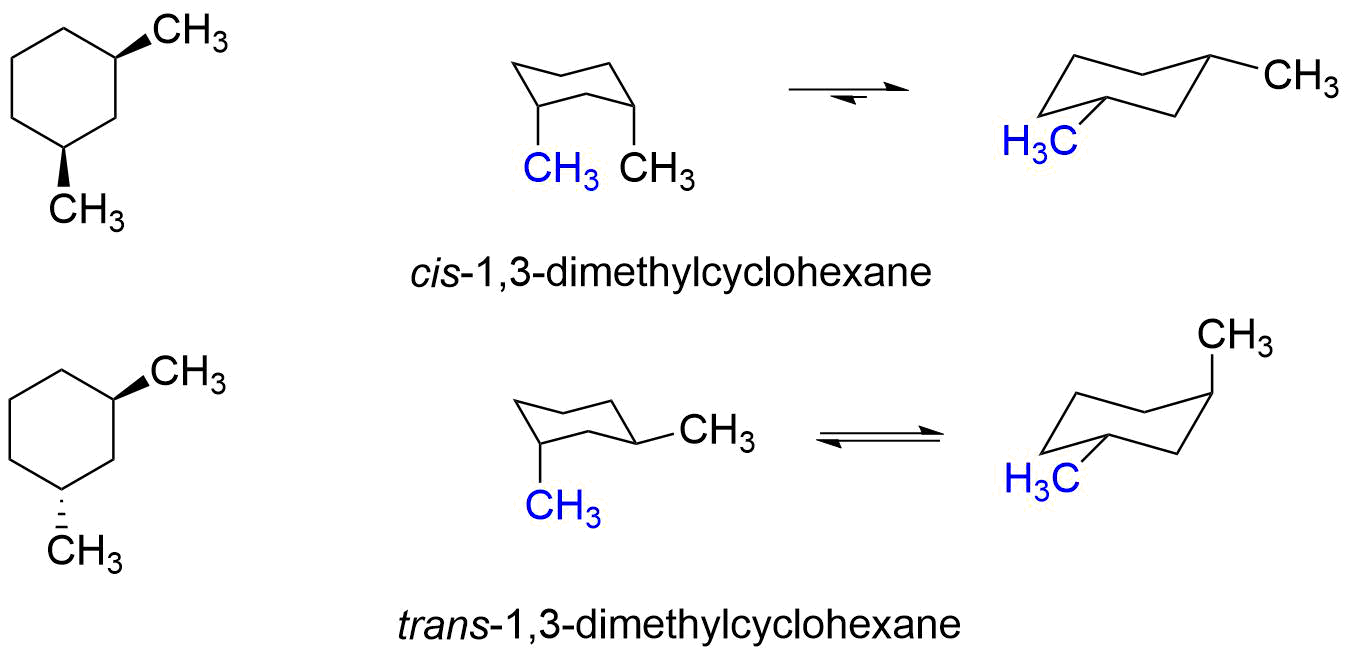 Source: chem.libretexts.org
Source: chem.libretexts.org
3 9 Conformations Of Disubstituted Cyclohexanes Chemistry Libretexts Axial and Equatorial Bonds in Cyclohexane. I was wondering whether Cis was with both being the same orientation or the same direction. In this video i explain how to draw the cis. Trans-13-Disubstituted cyclohexanes are like cis-12- and cis-14- and can flip between the two equivalent axialequatorial forms. In todays video I am going to be going over cis-trans stereiosiomers bicyclic molecules and how to draw out those chair conformations and determine their stability. A lot of students say Oh but theyre both equatorial so that means that they should be cis.
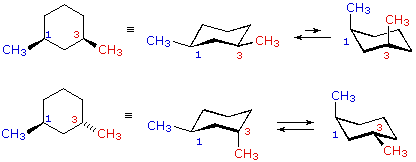 Source: chem.libretexts.org
Source: chem.libretexts.org
4 10 Conformations Of Disubstituted Cyclohexanes Chemistry Libretexts Hence the angle strain in the chair conformation is very small. S cis and s trans master organic chemistry. A lot of students say Oh but theyre both equatorial so that means that they should be cis. Which is more stable cis- or trans- isomers. If you are looking for Drawing chair conformations cis and trans youve come to the right place. Compounds with 13-axax-substitutions are always cis.
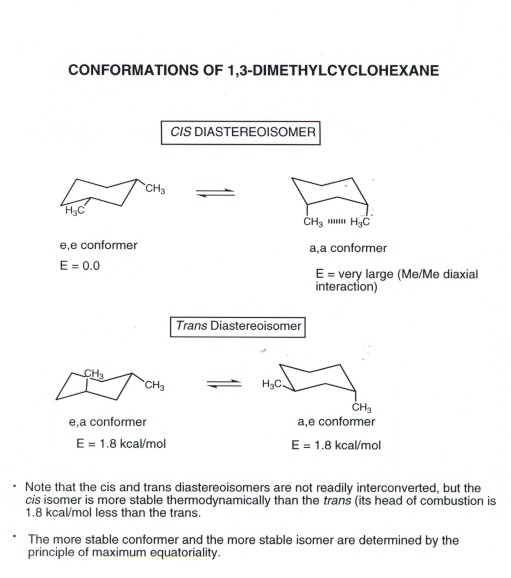 Source: research.cm.utexas.edu
Source: research.cm.utexas.edu
Cyclohexane Conformational Analysis Chair conformations Cis and Trans. Therefore in the chair-chair form of trans decalin the arrangement of the substituent was fixed whereas they are relatively flexible in the chair form of the cis decalin was and the conversion of two rings at once occurs fairly easily. The dihedral angle between two hydrogen atoms on adjacent carbon atoms on the same side of the ring is 55º. In the given figure various possible chair conformations of 12-dimethylcyclohexane are drawn. S cis and s trans master organic chemistry. The phenyl group locks the conformation of epoxide since it stays equatorial so there is only one conformation in each of the two isomers.
 Source: youtube.com
Source: youtube.com
How To Determine The Stability Of Cis Trans Cyclohexane Derivatives Baesed On Chair Conformations Youtube We have collect images about Drawing chair conformations cis and trans including i. Recall that diastereomers are non superimposable non mirror images. How to draw a chair conformation from cyclohexane how to distinguish between cis and tran chair conformation and problems dealing with chair conformation. Draw all the chair conformators of each isomer and decide that it is the most stable. Thats not how you decide it at all. S cis and s trans master organic chemistry.
 Source: chem.libretexts.org
Source: chem.libretexts.org
4 10 Conformations Of Disubstituted Cyclohexanes Chemistry Libretexts Energy difference values experimentally determined between two chair forms of carious groups to calculate the ratio of the two conformations in solution. There are two possibilities that are cis or trans but the position of the methyl group on axial or equatorial bond on cyclohexane determines whether the compound is cis or trans. I was wondering whether cis was with both being the same orientation or the same direction. Draw the chair conformations of. In the cases above the ring opens out to give a diaxial chair conformation. How To Determine The Stability Of A Declin 3 2 Conformations Of Cyclic Organic.
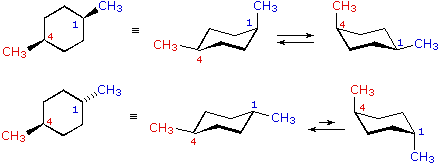 Source: chem.libretexts.org
Source: chem.libretexts.org
4 10 Conformations Of Disubstituted Cyclohexanes Chemistry Libretexts This means that the nucleophile and the epoxide oxygen will always be trans in the product. A Draw the most stable chair conformation for each of the compounds shown below. C The higher energy chair conformation contains two equatorial ethyl groups. Compounds with 13-eqeq-substitutions are always cis as well. The most stable conformation of the cyclohexane ring is called the chair conformation. S cis and s trans master organic chemistry.
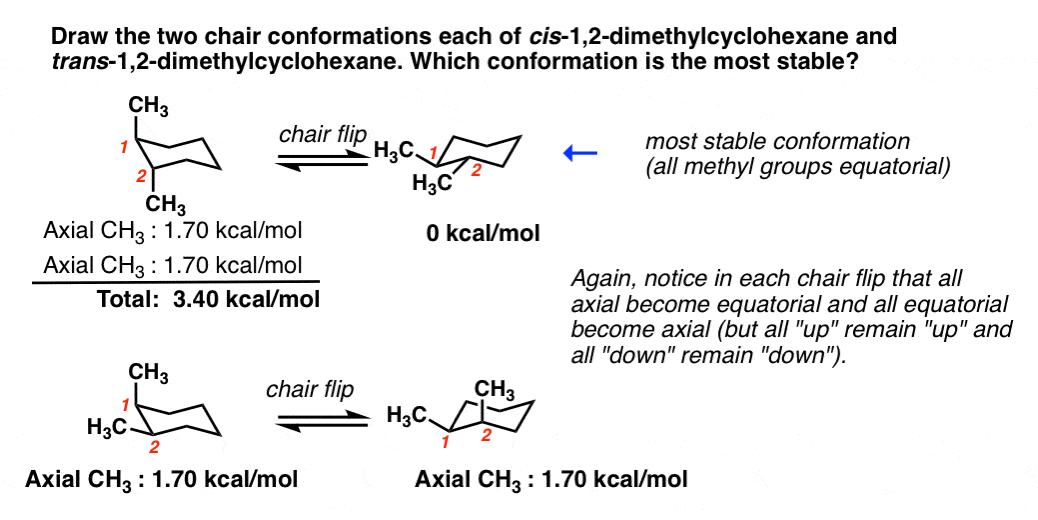 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Cyclohexane Chair Conformation Stability Which One Is Lower Energy We have collect images about Drawing chair conformations cis and trans including i. Which is more stable cis- or trans- isomers. Or one is equatorial and one is axial so they should be trans Wrong. Furthermore since 14-axax-bonds are always trans opposite direction. Draw the chair conformations of. Trans-13-Disubstituted cyclohexanes are like cis-12- and cis-14- and can flip between the two equivalent axialequatorial forms.
 Source: clutchprep.com
Source: clutchprep.com
For Cis 1 3 Dimethylcyclohexane Which Two Clutch Prep The azide N 3- nucleophile must attack the epoxide from behind in order to interact with the C-O σ orbital. The ground state conformation of cyclohexane is a fully staggered conformation which is shaped somewhat like. There are two possibilities that are cis or trans but the position of the methyl group on axial or equatorial bond on cyclohexane determines whether the compound is cis or trans. 2 Cis -14-Di- tert -butylcyclohexane has an axial tert -butyl group in the chair conformation and conversion to the twist-boat conformation places both groups in more favorable equatorial positions. Ok so I am going to start out with discussing cis-decalin and trans-decalin which are diastereomers a type of stereoisomers. The most stable conformation of the cyclohexane ring is called the chair conformation.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
How To Identify Cis And Trans Forms Of Cyclohexane Chemistry Stack Exchange Trans-decalin is a kind of rigid system and not like cyclohexane both the rings of the trans-decalin cannot be flip from one form of a chair into another form. In the given figure various possible chair conformations of 12-dimethylcyclohexane are drawn. Ok so I am going to start out with discussing cis-decalin and trans-decalin which are diastereomers a type of stereoisomers. I was wondering whether Cis was with both being the same orientation or the same direction. The most stable conformation of the cyclohexane ring is called the chair conformation. A The two chair conformations are equal in energy.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Cyclohexane Chair Conformation Stability Which One Is Lower Energy But they dont have to. Draw the chair conformations of. Axial and Equatorial Bonds in Cyclohexane. A The two chair conformations are equal in energy. The dihedral angle between two hydrogen atoms on adjacent carbon atoms on the same side of the ring is 55º. Trans-13-Disubstituted cyclohexanes are like cis-12- and cis-14- and can flip between the two equivalent axialequatorial forms.
 Source: differencebetween.com
Source: differencebetween.com
Difference Between Cis And Trans Cyclohexane Compare The Difference Between Similar Terms We have collect images about Drawing chair conformations cis and trans including i. Ok so I am going to start out with discussing cis-decalin and trans-decalin which are diastereomers a type of stereoisomers. 14 Which of the statements below correctly describes the chair conformations of trans-13-diethylcyclohexane. Cis and trans is actually going to be based on whether the groups are facing the same face of the ring. Lets get some practice drawing it chair confirmations one way to do it is to start by drawing two parallel lines that are offset from each others let me go ahead and show you what I mean so heres heres one line and then here is another line theyre parallel to each other but theyre offset a little bit next were going to draw two horizontal dotted lines so the top horizontal dotted line is going to be on level with that top. Trans-13-Disubstituted cyclohexanes are like cis-12- and cis-14- and can flip between the two equivalent axialequatorial forms.
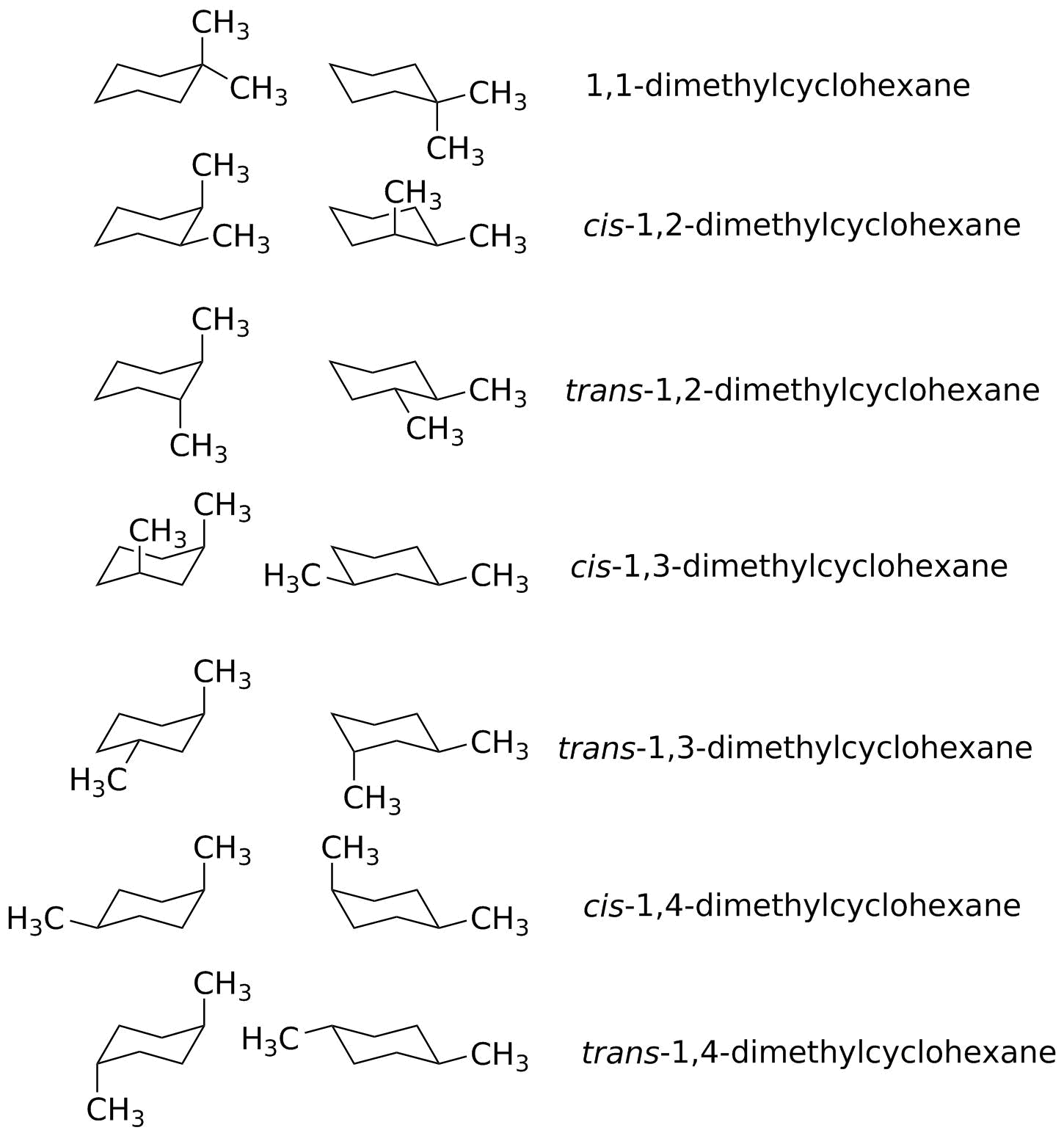 Source: sites.science.oregonstate.edu
Source: sites.science.oregonstate.edu
Chapter 4 Worked Problem 1 The chair forms are started for you and are labeled A B C D. The tetrahydronaphthyl moiety exists in an equatorial conformation with respect to the piperidine ring which exists in the chair conformation. The orientation of the N-phenyl group with respect to the N-acyl moiety is essentially invariant with an approximately 90 degrees dihedral angle. I was wondering whether cis was with both being the same orientation or the same direction. Or one is equatorial and one is axial so they should be trans Wrong. C The higher energy chair conformation contains two equatorial ethyl groups.
 Source: youtube.com
Source: youtube.com
Cis And Trans Substituent Relationships Organic Chemistry I Youtube I was wondering whether cis was with both being the same orientation or the same direction. Chair concordances can present the challenge in organic chemistry. Cis and trans chair conformations Warning. Select the more stable conformation for each compound. We have collect images about Drawing chair conformations cis and trans including i. The phenyl group locks the conformation of epoxide since it stays equatorial so there is only one conformation in each of the two isomers.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
How To Identify Cis And Trans Forms Of Cyclohexane Chemistry Stack Exchange Recall that diastereomers are non superimposable non mirror images. Energy difference values experimentally determined between two chair forms of carious groups to calculate the ratio of the two conformations in solution. Why is it called chair conformation. This means that the nucleophile and the epoxide oxygen will always be trans in the product. Cis and trans is actually going to be based on whether the groups are facing the same face of the ring. A particularly important case comes up with.

2 Which is more stable cis- or trans- isomers. We will also discuss the relationship between cistrans and axialequatorial. The most stable conformation of the cyclohexane ring is called the chair conformation. Can it still be Cis with a an equatorial up and equatorial down or would that be considered Trans. Why is it called chair conformation. For the cis compounds a chair conformation in which the rotation of at least one of the substituents is hindered explains the experimental data PMR CMR and IR.
 Source: onlineorganicchemistrytutor.com
Source: onlineorganicchemistrytutor.com
Possible Chair Conformations Of 1 2 Dimethylcyclohexane However in the Na-methyl series 1a 1b 2a 2b both the cis 1b 2b and trans 1a 2a diastereomers existed in the chair conformation to relieve the A12-strain between the Na-methyl function and the substituent at C1. The orientation of the N-phenyl group with respect to the N-acyl moiety is essentially invariant with an approximately 90 degrees dihedral angle. 14 Which of the statements below correctly describes the chair conformations of trans-13-diethylcyclohexane. Can it still be Cis with a an equatorial up and equatorial down or would that be considered Trans. The ground state conformation of cyclohexane is a fully staggered conformation which is shaped somewhat like. The most stable conformation of the cyclohexane ring is called the chair conformation.
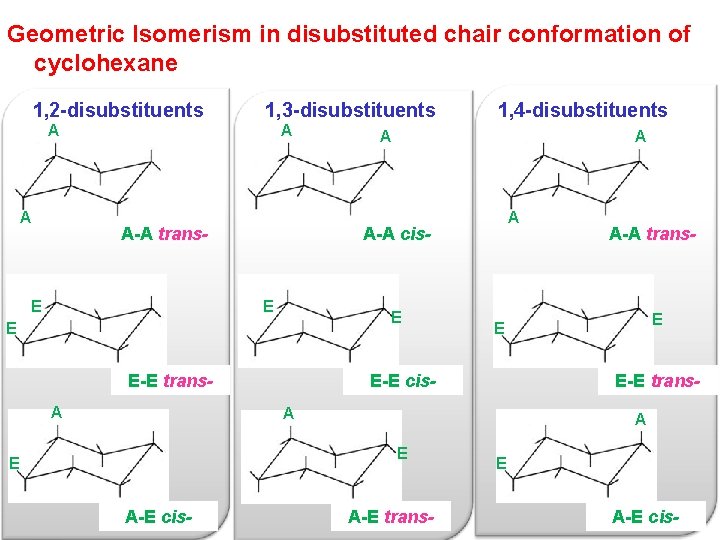 Source: slidetodoc.com
Source: slidetodoc.com
Chapter 4 Alkanes Alkenes And Alkynes Nomenclature Conformational Three-dimensional aspects of molecules Conformation. A particularly important case comes up with. Possible chair conformations of 12-dimethylcyclohexane. The phenyl group locks the conformation of epoxide since it stays equatorial so there is only one conformation in each of the two isomers. Select the more stable conformation for each compound. This means that the nucleophile and the epoxide oxygen will always be trans in the product.
 Source: youtube.com
Source: youtube.com
Determining Cis Trans On Cyclohexanes Youtube The propanilido group is also equatorial and antiperiplanar. The orientation of the N-phenyl group with respect to the N-acyl moiety is essentially invariant with an approximately 90 degrees dihedral angle. Draw the chair conformations of. However in the Na-methyl series 1a 1b 2a 2b both the cis 1b 2b and trans 1a 2a diastereomers existed in the chair conformation to relieve the A12-strain between the Na-methyl function and the substituent at C1. Chair concordances can present the challenge in organic chemistry. S cis and s trans master organic chemistry.
Please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark or be able to bookmark this blog page in this website. Thank you …










