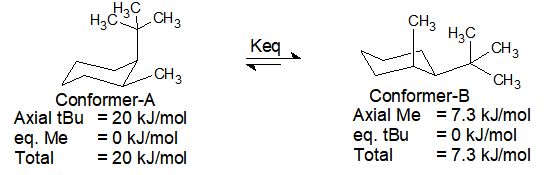
Chair conformation equilibrium Alternate your axial substituents up and down all the way around your cyclohexane. There is so little of the conformation with an axial tert.
Chair Conformation Equilibrium, The larger the size of the substituent the larger the energy difference and the equilibrium constant K so the equilibrium lies more. But notice what has happened to the hydrogens. In real sense the cyclohexanes adopts two conformations.
 Group Theory Organic Chemistry Chemistry Classroom Chemistry Lessons From pinterest.com
Group Theory Organic Chemistry Chemistry Classroom Chemistry Lessons From pinterest.com
Another Article :
Chemistry questions and answers. For most classes all you will need to know how to do is use equatorial preference to predict the most stable chair conformation. And now the stabilities. Draw the second chair conformation ring-flip-check this post if not sure. In this process the equa-torial hydrogens have become axial and the axial hydrogens have become equatorial.
This is a multistep process so here Im going to walk you through it from scratch.
1 1 4 4 In the illustration above the two chair conformations are in equilibrium. Hence the chair conformation in which the tert-butyl group assumes the equatorial position is overwhelmingly favored. What is interesting here is that this equilibrium is not a 50. In this process the equa-torial hydrogens have become axial and the axial hydrogens have become equatorial. When one chair conformation flips into the other the axial and equatorial hydrogens interconvert.
 Source: pinterest.com
Source: pinterest.com
Kinetics Rate Laws Science Chemistry Chemistry Education Chemistry Called chair flipping creating 2 conformations for the same chair. What is interesting here is that this equilibrium is not a 50. A common exam question tests the. What is the reason for this. Below the figure shows the chair conformations of cis-1 3-dimethylcyclohexane. Choose the lowest energy conformation for the following compound.
 Source: pinterest.com
Source: pinterest.com
Organic Chemistry Nomenclature Organic Chemistry Nomenclature Chemistry Organic Che Organic Chemistry Cheat Sheet Organic Chemistry Notes Chemistry Notes It is shifted to the more stable chair conformation. For each chair conformer add the energy of all the groups on axial position. Although the hydrocarbon cyclohexane is typically drawn as if it were flat in reality the structure is not flat at all. The absence of angle strain is a result of cyclohexanes bond angle being very close to the ideal tetrahedral bond angle of 1095 in its chair conformer. So far we have not described in detail the rotational journey of the cyclohexane and what is the final result. The different conformations are called conformers a blend of the words conformation and isomer.
 Source: in.pinterest.com
Source: in.pinterest.com
Cyclohexane And Chair Flipping Intermediate Boat Conformation Organic Chemistry Video Organic Chemistry Chemistry Biochemistry Chemistry questions and answers. Most of the time the structure exists in what is called the chair conformation. Chemistry questions and answers. The different conformations are called conformers a blend of the words conformation and isomer. And now the stabilities. Choose the lowest energy conformation for the following compound.
 Source: pinterest.com
Source: pinterest.com
Organic Chemistry I Study Guides Organic Chemistry Organic Chemistry Study Chemistry Basics Choose the lowest energy conformation for the following compound. The first stage involves the transition from an equilibrium half-chair conformation to a twist-boat conformation with a significant increase in energy. The chair conformation is the most stable form of cyclohexane due to the absence of angle and torsional strain. Below the figure shows the chair conformations of cis-1 3-dimethylcyclohexane. If I need to give more information let me know. The absence of angle strain is a result of cyclohexanes bond angle being very close to the ideal tetrahedral bond angle of 1095 in its chair conformer.
 Source: pinterest.com
Source: pinterest.com
Group Theory Organic Chemistry Chemistry Classroom Chemistry Lessons In the chair conformation four carbon atoms are coplanar and the other two are puckered out of this plane one being puckered up above this planethe carbon at the extreme right of the depicted chair form and one being puckered down out of this planethe one at the extreme left of the illustration. Chair conformations in equilibrium You should be able to quickly draw cyclohexane rings in which the axial and equatorial ties are easily identifiable and distinguishable. And now the stabilities. This conformation is called the chair because it looks sort of like a reclining lounge chair as shown here. What is interesting here is that this equilibrium is not a 50. The different conformations are called conformers a blend of the words conformation and isomer.
 Source: pinterest.com
Source: pinterest.com
Video How To Draw Cyclohexane Chair Conformations And Ring Flips Organicchemistry Chemistry Education Organic Chemistry Online Tutoring Below the figure shows the chair conformations of cis-1 3-dimethylcyclohexane. Therefore it is common for the equilibrium to favor one side or the other. Draw the second chair conformation ring-flip-check this post if not sure. This conformation is called the chair because it looks sort of like a reclining lounge chair as shown here. The absence of angle strain is a result of cyclohexanes bond angle being very close to the ideal tetrahedral bond angle of 1095 in its chair conformer. Chair conformation equilibrium The various conformations of cyclohexane are m rapid equilibrium with one another but at any moment almost all of the molecules exist m the chair conformation Not more than one or two molecules per thousand are present m the skew boat confer matron Thus the discussion of cyclohexane conformational analysis that follows focuses exclusively on the chair conformation.
 Source: pinterest.com
Source: pinterest.com
Antiperiplanar Relationships The E2 Reaction And Cyclohexane Rings Organic Chemistry Organic Chemistry Study Chemistry Lessons When one chair conformation flips into the other the axial and equatorial hydrogens interconvert. As we can see from the above calculations as the temperature rises the percentage contribution by equatorial conformation decreases while that by axial conformation. The chair conformation is the most stable form of cyclohexane due to the absence of angle and torsional strain. Chemistry questions and answers. 5 in favor of the one where the methyl group is equatorial. A B C D E F.
 Source: pinterest.com
Source: pinterest.com
Organic Chemistry Educational Infographic Cyclohexane And Chair Conformation Video Organic Chemistry Chemistry Educational Infographic Ah I I 10. 5 in favor of the one where the methyl group is equatorial. At 25 C 9999 of all molecules in a cyclohexane solution adopt this conformation. For most classes all you will need to know how to do is use equatorial preference to predict the most stable chair conformation. Although the hydrocarbon cyclohexane is typically drawn as if it were flat in reality the structure is not flat at all. The chair conformation is the most stable conformer.
 Source: pinterest.com
Source: pinterest.com
D And L Notation For Sugars And Amino Acids Chemistry Notations Organic Chemistry The boat conformation feels some what greater sterric hindrance so it is less often found while the chair conformation is very stable hence it is favored. The two conformations exist in equilibrium but often dont have the same energy as one another. Of the rightmost carbon changes one chair conformation into another completely equivalent chair conformation. You may remember from first semester organic chemistry that the lowest energy conformation of cyclohexane is the chair. So far we have not described in detail the rotational journey of the cyclohexane and what is the final result. The chair conformation has alternating axial up axial down so once you have that single axial substituent move on to.
 Source: pinterest.com
Source: pinterest.com
T Butyl Cycohexane 1 3 Diaxial Interactions Interactive Methyl Group Notations Alternate your axial substituents up and down all the way around your cyclohexane. Choose the lowest energy conformation for the following compound. Below the figure shows the chair conformations of cis-1 3-dimethylcyclohexane. But notice what has happened to the hydrogens. Calculate the difference in the Gibbs free energy between the second and first conformation including the algebraic sign. 5 in favor of the one where the methyl group is equatorial.
 Source: pinterest.com
Source: pinterest.com
I Guess It S Casual Friday For Some Molecules Chemistry Jokes Chemistry Humor Science Humor When one chair conformation flips into the other the axial and equatorial hydrogens interconvert. Every carbon on the chair conformation has. Ah I I 10. The different conformations are called conformers a blend of the words conformation and isomer. In ad-dition up carbons have become down carbons and vice versa. The methyl group is thus forced to take up the axial position.
 Source: pinterest.com
Source: pinterest.com
Pin On Chemistry Every carbon on the chair conformation has. The chair conformation is the most stable form of cyclohexane due to the absence of angle and torsional strain. Choose the lowest energy conformation for the following compound. Thus we can say that at 150C 888 of equatorial conformation and 112 of axial conformation exists in equilibrium. Identify the equilibrium below that shows the two chair conformations of the following compound. The chair conformation has alternating axial up axial down so once you have that single axial substituent move on to.
 Source: pinterest.com
Source: pinterest.com
Sodium Borohydride Nabh4 Reduction Reaction Mechanism Organic Chemistry Study Organic Chemistry Reactions Organic Chemistry It is shifted to the more stable chair conformation. Thus we can say that at 150C 888 of equatorial conformation and 112 of axial conformation exists in equilibrium. For most classes all you will need to know how to do is use equatorial preference to predict the most stable chair conformation. For cis-13-dimethylcyclohexane which two chair conformations are in equilibrium. 50 mixture of the conformations. Below the figure shows the chair conformations of cis-1 3-dimethylcyclohexane.
 Source: pinterest.com
Source: pinterest.com
Macromolecules Biochemistry Energy Storage Most of the time the structure exists in what is called the chair conformation. A common exam question tests the. Of the rightmost carbon changes one chair conformation into another completely equivalent chair conformation. The chair conformation has alternating axial up axial down so once you have that single axial substituent move on to. Called chair flipping creating 2 conformations for the same chair. The first stage involves the transition from an equilibrium half-chair conformation to a twist-boat conformation with a significant increase in energy.
 Source: pinterest.com
Source: pinterest.com
Cyclohexane Chair Conformation Stability Which One Is Lower Energy Science Notes Chemistry Notes Organic Chemistry Notes Ah I I 10. Most of the time the structure exists in what is called the chair conformation. This is a multistep process so here Im going to walk you through it from scratch. In the chair conformation four carbon atoms are coplanar and the other two are puckered out of this plane one being puckered up above this planethe carbon at the extreme right of the depicted chair form and one being puckered down out of this planethe one at the extreme left of the illustration. Identify the equilibrium below that shows the two chair conformations of the following compound. A boat conformation and a chair conformation.
 Source: pinterest.com
Source: pinterest.com
6 1b Organic Chemistry Biochemistry Various Organic Functional Group Cset Study Gu Functional Groups Organic Chemistry Functional Group Organic Chemistry The larger the size of the substituent the larger the energy difference and the equilibrium constant K so the equilibrium lies more. The equilibrium will tend to lie toward the more stable chair conformation. Alternate your axial substituents up and down all the way around your cyclohexane. The chair conformation is the most stable form of cyclohexane due to the absence of angle and torsional strain. At 25 C 9999 of all molecules in a cyclohexane solution adopt this conformation. Number the ring and draw any chair conformation of the compound.
 Source: pinterest.com
Source: pinterest.com
D And L Notation For Sugars And Amino Acids Chemistry Notations Organic Chemistry Called chair flipping creating 2 conformations for the same chair. For each chair conformer add the energy of all the groups on axial position. The larger the size of the substituent the larger the energy difference and the equilibrium constant K so the equilibrium lies more. For cis-13-dimethylcyclohexane which two chair conformations are in equilibrium. The ratio of two chair conformations in the following mixture is 95. Identify the equilibrium below that shows the two chair conformations of the following compound.
 Source: pinterest.com
Source: pinterest.com
Conformations Of Cyclohexanes Conformations Of Cyclohexanes Mcat Mcatprep Mcatstudying Organicchemistry Organicchem Organik Kimya Biyokimya Kimya The absence of angle strain is a result of cyclohexanes bond angle being very close to the ideal tetrahedral bond angle of 1095 in its chair conformer. A B C D E F. Given your value in a calculate the percent of the chair indicated as B presented in an equilibrium mixture of the conformers at 25ºC. The methyl group is thus forced to take up the axial position. In ad-dition up carbons have become down carbons and vice versa. Choose the lowest energy conformation for the following compound.
Please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark or be able to bookmark this blog page in this website. Thank you …