Chair conformation for a disubstituted cyclohexane Which of the following is the correct chair representation of the disubstituted cyclohexane in its lowest energy conformation. Cyclohexane is unique in being the only cyclic hydrocarbon which is.
Chair Conformation For A Disubstituted Cyclohexane, All cyclohexanes have two chair conformations. However sometimes you will be required to use energetics to calculate the exact percentages of each chair in solution. This is a multistep process so here Im going to walk you through it from scratch.
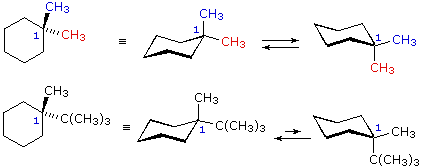 4 10 Conformations Of Disubstituted Cyclohexanes Chemistry Libretexts From chem.libretexts.org
4 10 Conformations Of Disubstituted Cyclohexanes Chemistry Libretexts From chem.libretexts.org
Another Article :
For the cyclohexane ring itself the two conformers from the ring flipping are equivalent in terms of energy since there are always six hydrogens in axial position and six hydrogens in equatorial position. This means there are two possible forms for each cis-trans isomer. At each position one substituent is axial loosely perpendicular to the ring and one is equatorial loosely in the plane of the ring. Disubstituted cyclohexanes retain their cis- or trans- conformations even after a ring flip. Lets see the example of methylcyclohexane.
There is only one chair conformation of cis-14-dimethylcyclohexane.
Circle the most stable of the two. We start with the notion that the conformation of cyclohexane derivatives is based on the chair. The chair form The structure of cis-14-dimethylcyclohexane is You can draw two flipped cyclohexane chairs. A Draw the two possible chair confomations for the tri-substituted cyclohexane shown below. There is only one chair conformation of cis-14-dimethylcyclohexane.
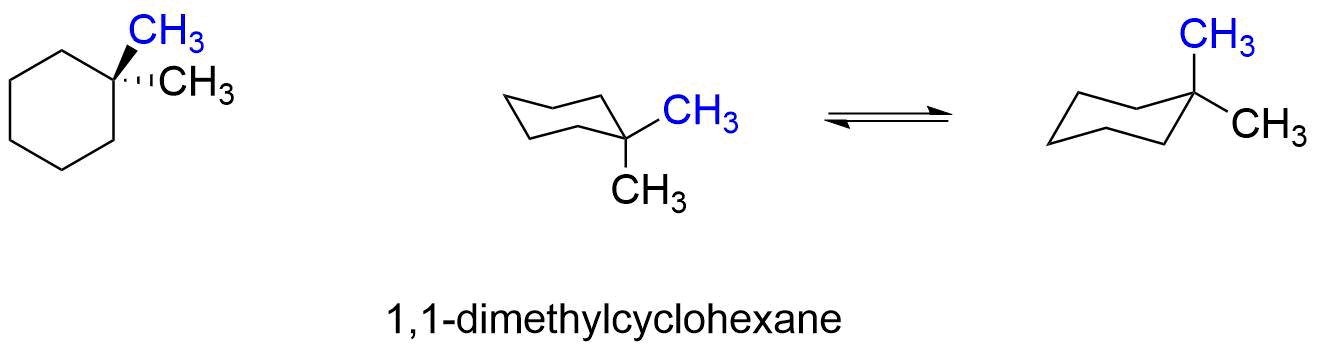 Source: chem.libretexts.org
Source: chem.libretexts.org
3 9 Conformations Of Disubstituted Cyclohexanes Chemistry Libretexts Lets look at cis- isomers first. Once youve examined the preceding points practice drawing some cyclohexane rings in the two perspec-tives. In a cis-disubstituted cyclohexane two substituents remain present on the same side of the peripheral plane of the carbon ring. The chair conformation has alternating axial up axial down so once you have that single axial substituent move on to. Lets see the example of methylcyclohexane. Two in another plane in front of the paper and the remaining two in a.
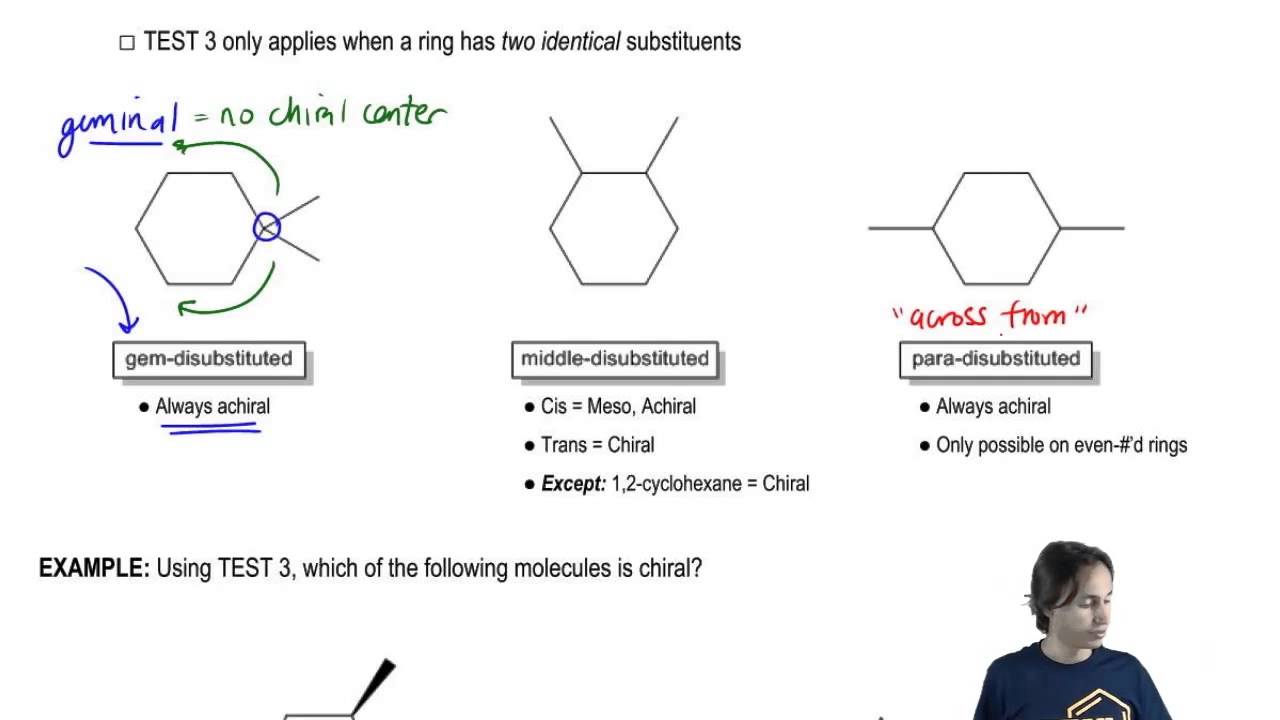 Source: youtube.com
Source: youtube.com
Three Types Of Disubstituted Cycloalkanes Youtube In a cis-disubstituted cyclohexane two substituents remain present on the same side of the peripheral plane of the carbon ring. Which of the following is the correct chair representation of the disubstituted cyclohexane in its lowest energy conformation. For substituted cyclohexane however the two chair conformations are not equivalent any more. Draw two templates that represents the two chair conformations of cyclohexane and number the carbon atoms. There are one chair conformation and two boat conformations of cis-14-dimethylcyclohexane. The chair form The structure of cis-14-dimethylcyclohexane is You can draw two flipped cyclohexane chairs.
 Source: pinterest.com
Source: pinterest.com
Conformers Of Cyclohexane Stability And Energy Level Ring Flipping Mon Energy Level Stability Energy There are one chair conformation and two boat conformations of cis-14-dimethylcyclohexane. The different conformations are called conformers a blend of the words conformation and isomer. Every carbon on the chair conformation has 1 substituent. Cyclohexane and the Chair Structure. Lets see the example of methylcyclohexane. It is important for you to be able to draw a cyclohexane chair conformation.
 Source: youtube.com
Source: youtube.com
06 06 Disubstituted Cyclohexanes And Beyond Youtube With no torsional strain and no angle strain cyclohexane is the most stable of all the small rings of. Two in another plane in front of the paper and the remaining two in a. This means there are two possible forms for each cis-trans isomer. As a general rule the most stable chair conformation of a six-membered ring will be that in which the bulkiest groups are in the equatorial position. Draw the corresponding planar overhead representation using wedges and hashed bonds to indicate the substituent positions. For convenience you may abbreviate the substituents Me Et Pr Bu iPr tBu or the like.
 Source: chem.libretexts.org
Source: chem.libretexts.org
4 10 Conformations Of Disubstituted Cyclohexanes Chemistry Libretexts Disubstituted cyclohexanes conformation If a disubstituted cyclohexane has two different substituents then the most stable conformation is the chair that has the larger substituent m an equatorial orientation This IS most apparent when one of the substituents is a bulky group such as tert butyl Thus the most stable conformation of cis 1 tert butyl 2 methylcyclohexane has an equatorial tert. Two in another plane in front of the paper and the remaining two in a. The chair conformation can be viewed as having two carbon atoms on the plane of the paper. All cyclohexanes have two chair conformations. Once youve examined the preceding points practice drawing some cyclohexane rings in the two perspec-tives. The following is the correct way to draw chair cyclohexane.
 Source: slidetodoc.com
Source: slidetodoc.com
4 13 Disubstituted Cyclohexane When Multiple Substituents Are At each position one substituent is axial loosely perpendicular to the ring and one is equatorial loosely in the plane of the ring. Circle the more stable one. For substituted cyclohexane however the two chair conformations are not equivalent any more. Correct incorrect a a e e a a e e a a e. There is only one chair conformation of cis-14-dimethylcyclohexane. Every carbon on the chair conformation has 1 substituent.
 Source: youtube.com
Source: youtube.com
Conformational Analysis Of Disubstituted Cyclohexane Stereochemistry Organic Chemistry Youtube Cyclohexane and the Chair Structure. The chair conformation can be viewed as having two carbon atoms on the plane of the paper. The chair form The structure of cis-14-dimethylcyclohexane is You can draw two flipped cyclohexane chairs. Clearly Indicate whether the substituents are axial a or equatorial e. There is only one chair conformation of cis-14-dimethylcyclohexane. 6 points 03 b Using Newman projection formulas draw the two possible chair conformations for the.
 Source: pinterest.com
Source: pinterest.com
Basicity Of Disubstituted Aromatic Amines Resonance Mesomeric Effect Organic Chemistry Organic Chem Basic Facts Cyclohexane structure that you draw. The chair structure of cyclohexane is considered to be the perfect conformation. The chair conformation has alternating axial up axial down so once you have that single axial substituent move on to. Once youve examined the preceding points practice drawing some cyclohexane rings in the two perspec-tives. The two conformations are identical. Circle the more stable one.
 Source: youtube.com
Source: youtube.com
Conformational Analysis Of Disubstituted Cyclohexane Stereochemistry Organic Chemistry Youtube The different conformations are called conformers a blend of the words conformation and isomer. Draw the corresponding planar overhead representation using wedges and hashed bonds to indicate the substituent positions. Draw two templates that represents the two chair conformations of cyclohexane and number the carbon atoms. For most classes all you will need to know how to do is use equatorial preference to predict the most stable chair conformation. Disubstituted cyclohexanes retain their cis- or trans- conformations even after a ring flip. It doesnt matter which chair conformation is drawn first because the chair intercon-version does not affect the trans relationship of the two methyl groups.
 Source: ar.pinterest.com
Source: ar.pinterest.com
Pin On Tannins Polyphenols Draw structures of the two chair conformations of trans-13-dimethylcyclohexane. Every carbon on the chair conformation has 1 substituent. Which of the following is the correct chair representation of the disubstituted cyclohexane in its lowest energy conformation. Use the following three steps. To be graded properly include the hydrogen atoms on the chirality centers asymmetric carbons. Cyclohexane structure that you draw.
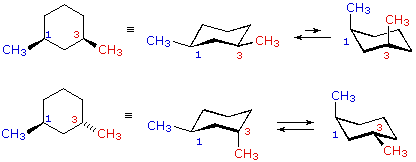 Source: chem.libretexts.org
Source: chem.libretexts.org
4 10 Conformations Of Disubstituted Cyclohexanes Chemistry Libretexts Cyclohexane structure that you draw. Use the following three steps. A Draw the two possible chair confomations for the tri-substituted cyclohexane shown below. A trisubstituted cyclohexane compound is given below in its chair conformation. This means there are two possible forms for each cis-trans isomer. Every carbon on the chair conformation has 1 substituent.
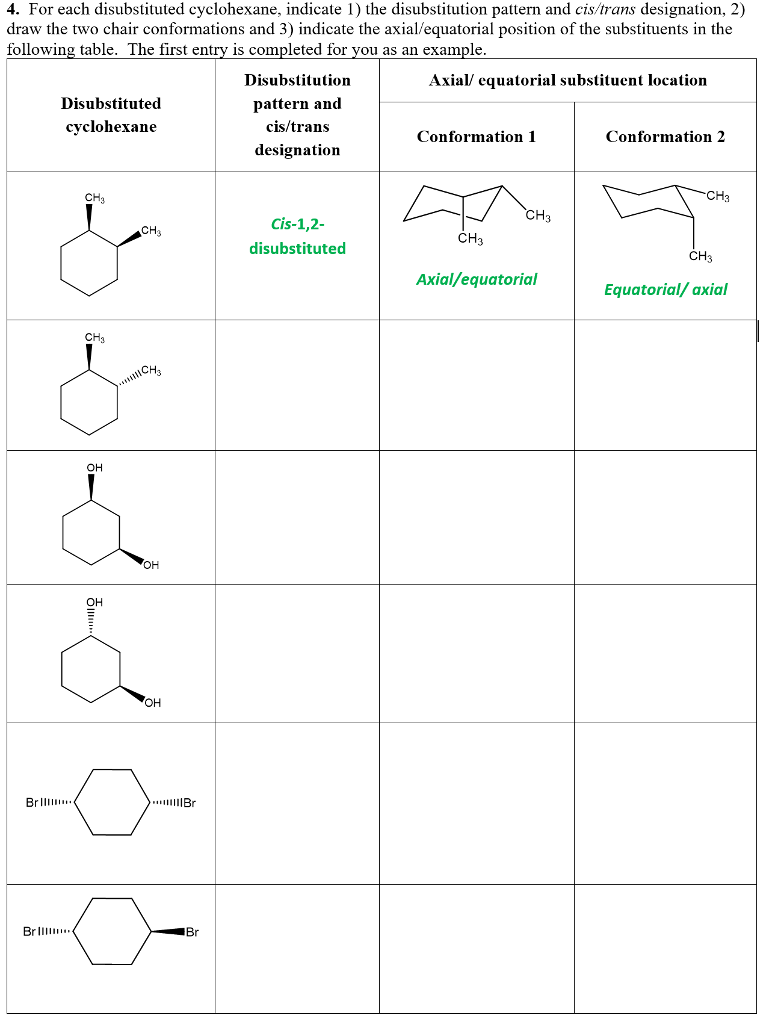 Source: chegg.com
Source: chegg.com
Solved 4 For Each Disubstituted Cyclohexane Indicate 1 Chegg Com As a general rule the most stable chair conformation of a six-membered ring will be that in which the bulkiest groups are in the equatorial position. Based on this we can predict that the conformer which places both substituents equatorial will be the more stable conformer. The following is the correct way to draw chair cyclohexane. The chair structure consists of a six-membered ring where every C-C bond exists in a staggered conformation. Circle the more stable one. The chair form The structure of cis-14-dimethylcyclohexane is You can draw two flipped cyclohexane chairs.
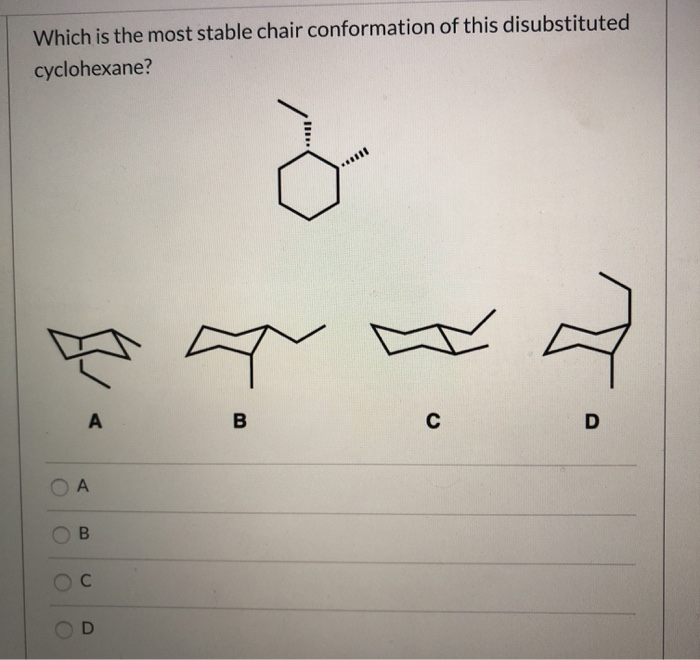 Source: chegg.com
Source: chegg.com
Solved Which Is The Most Stable Chair Conformation Of This Chegg Com At each position one substituent is axial loosely perpendicular to the ring and one is equatorial loosely in the plane of the ring. To be graded properly include the hydrogen atoms on the chirality centers asymmetric carbons. It is important for you to be able to draw a cyclohexane chair conformation. Disubstituted cyclohexanes retain their cis- or trans- conformations even after a ring flip. With no torsional strain and no angle strain cyclohexane is the most stable of all the small rings of. The ground state conformation of cyclohexane is a fully staggered conformation which is shaped somewhat like a chair.
 Source: youtube.com
Source: youtube.com
Ozztube 8 Conformational Analysis Of Disubstituted Cyclohexanes Youtube The chair conformation is the most stable conformer. Two in another plane in front of the paper and the remaining two in a. In a cis-disubstituted cyclohexane two substituents remain present on the same side of the peripheral plane of the carbon ring. Solution In a trans-disubstituted cyclohexane the two substituent groups have an updown re-lationship. In this conformation there is no torsional strain at all and as we shall see later no strain of any kind. Which of the following is the correct chair representation of the disubstituted cyclohexane in its lowest energy conformation.
 Source: youtube.com
Source: youtube.com
Conformational Analysis Of Disubstituted Cyclohexane Stereochemistry Organic Chemistry Youtube Two in another plane in front of the paper and the remaining two in a. Step 1 Begin by drawing two parallel bonds slanted to the left for one perspective and slanted to the right for the other. There is only one chair conformation of cis-14-dimethylcyclohexane. A trisubstituted cyclohexane compound is given below in its chair conformation. All cyclohexanes have two chair conformations. It doesnt matter which chair conformation is drawn first because the chair intercon-version does not affect the trans relationship of the two methyl groups.
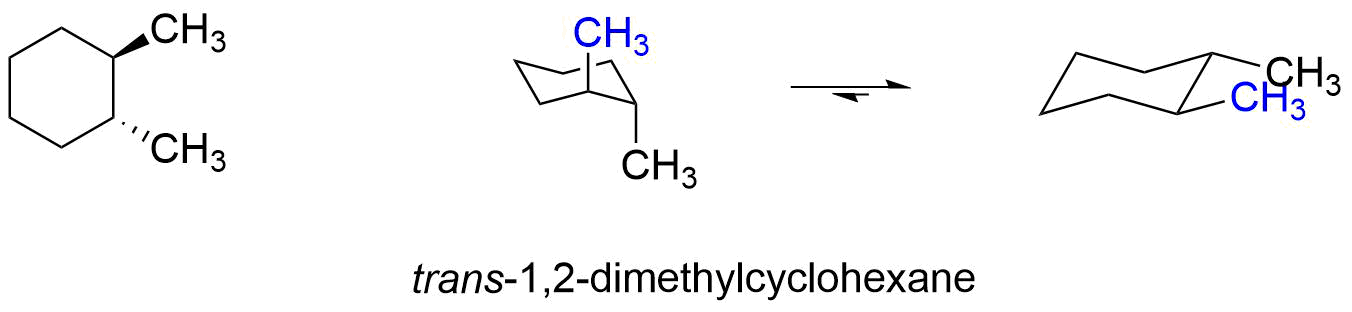 Source: chem.libretexts.org
Source: chem.libretexts.org
3 9 Conformations Of Disubstituted Cyclohexanes Chemistry Libretexts In a cis-disubstituted cyclohexane two substituents remain present on the same side of the peripheral plane of the carbon ring. There are one chair conformation and two boat conformations of cis-14-dimethylcyclohexane. The boat forms You can also draw two flipped boat conformations. However sometimes you will be required to use energetics to calculate the exact percentages of each chair in solution. For most classes all you will need to know how to do is use equatorial preference to predict the most stable chair conformation. A trisubstituted cyclohexane compound is given below in its chair conformation.
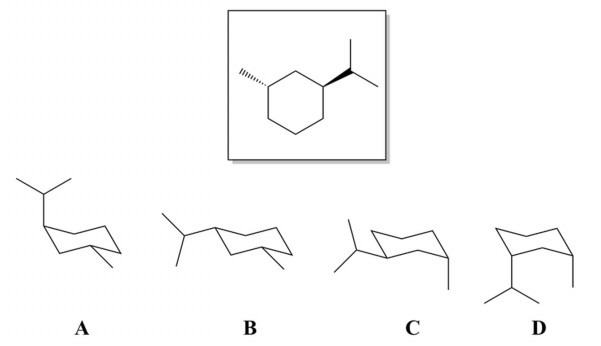 Source: chegg.com
Source: chegg.com
Solved 1 Which Of The Following Perspective Drawings Is The Chegg Com The chair conformation can be viewed as having two carbon atoms on the plane of the paper. The bond angle is very close to the ideal value. It has no torsional strain as all the C-H bonds are staggered to each other. For cyclohexanes you may be asked to draw a chair in which case all substituents must be either axial or equatorial. When choosing the most stable conformation we look at both cis- and trans- isomers separately. In the case of a disubstituted cyclohexane ring in which both substituents cannot be equatorial the lower energy conformation generally places the bulkier substituent in the equatorial position.
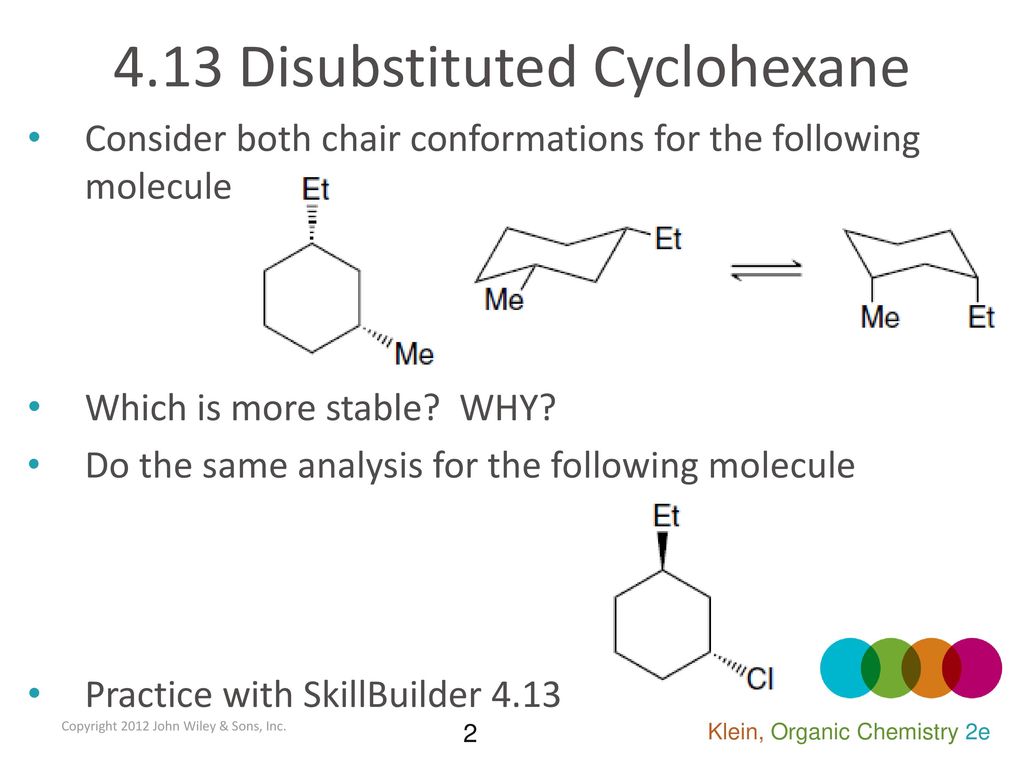 Source: slideplayer.com
Source: slideplayer.com
4 13 Disubstituted Cyclohexane Ppt Download At each position one substituent is axial loosely perpendicular to the ring and one is equatorial loosely in the plane of the ring. For cyclohexanes you may be asked to draw a chair in which case all substituents must be either axial or equatorial. The chair form The structure of cis-14-dimethylcyclohexane is You can draw two flipped cyclohexane chairs. However sometimes you will be required to use energetics to calculate the exact percentages of each chair in solution. Cyclohexane and the Chair Structure. We start with the notion that the conformation of cyclohexane derivatives is based on the chair.
Please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save or be able to bookmark this blog page in this website. Thank you …










