Chair conformation generator These are ready to be exploited for drug design in the presence and also in the absence of detailed knowledge about the three-dimensional structure of the protein active site. Other principal conformations of pyranoses are halfchair H boat B and skew S conformation which are named as indicated.
Chair Conformation Generator, Calculating Flip Energy. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. The key difference between chair and boat conformation is that chair conformation has low energy whereas boat conformation has high energy.
 Chair Conformations Examples Youtube From youtube.com
Chair Conformations Examples Youtube From youtube.com
Another Article :
Chair conformation lounge chair - used to kick back and relax. Driven by the wealth and diversity of bond angle and torsion information in the Cambridge Structural Database the CSD Conformer Generator produces realistic ensembles of low energy ligand structures. Ive drawn cis-14-di-tert-butyl-cyclohexane with ChemDraw and used EditGet 3D Model and ChemBioDraw gave me the structure which is NOT the energetical most suitable one. Explaining how A-Values are related to cyclohexane flip energy. These are ready to be exploited for drug design in the presence and also in the absence of detailed knowledge about the three-dimensional structure of the protein active site.
Number the ring and draw any chair conformation of the compound.
The C-C-C bonds are very close to 1095 o so it is almost free of angle strain. Next add a downward-pointing V tip to one end this is the tail of the chair. Structure and conformations of decalins. Worse its really difficult to show which substituents are axial vs equatorial. Explaining how A-Values are related to cyclohexane flip energy.
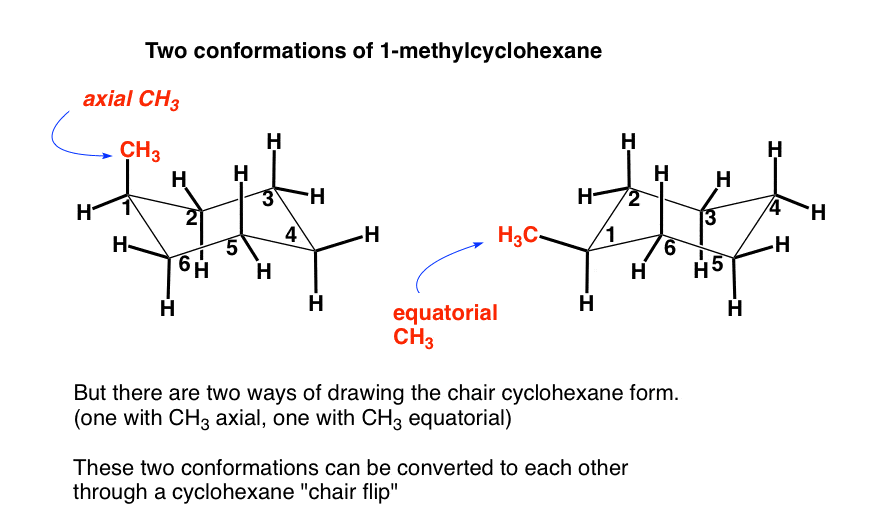 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
The Cyclohexane Chair Flip Master Organic Chemistry Cyclic alkanes can also interconvert between their conformers by rotation of the single carbon to carbon bonds. If you switch out a carbon atom for an oxygen atom and you are set to study the pyranoses of biochemistry eg. Calculating Flip Energy. This is where the messiness and confusion arises. Converting Cyclohexane Chair to Double Newman Projection Tutorial Video. Cyclic alkanes can also interconvert between their conformers by rotation of the single carbon to carbon bonds.
 Source: pinterest.com
Source: pinterest.com
Antiperiplanar Relationships The E2 Reaction And Cyclohexane Rings Organic Chemistry Organic Chemistry Study Chemistry Lessons 1 3 Dibromocyclohexane Chair Conformation. This chair-chair interconversion that leads to the generation of two equivalent energy forms is known as ring flipping. To draw the chair conformation of D-glucose you need to know the orientations of the axial and equatorial bonds on carbon atoms of the chair conformation. In each of the conformations drawn in step 4 circle the axial substituents other than hydrogen. If you switch out a carbon atom for an oxygen atom and you are set to study the pyranoses of biochemistry eg. For 1 3 disubstituted cyclohexanes the cis form is diequatorial and the flipped conformation suffers additional steric interaction between the two axial groups.
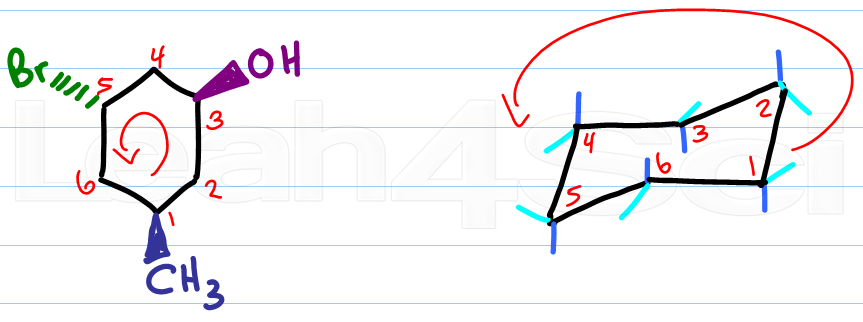 Source: leah4sci.com
Source: leah4sci.com
Drawing Chair Conformations And Ring Flips For Cyclohexane In Organic Chemistry The A-value of a tert-Butyl is in the range of 5 kcalmol which is around the value of the energy difference between cyclohexane in twist-boat and chair conformation. This question takes a complicated substituent filled Chair Conformation and transforms it into a Newman Projection. A chair conformation of cyclohexane can undergo a conformational change into another chair conformer by the partial rotation of C-C bonds. Finally add an upward-pointing V tip to the other end this is the nose of the chair. Fischer projection for the open-chain molecules Haworth projections focusing on cyclic pyranoses and your regular chair conformations. Structure and conformations of decalins.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Cyclohexane Chair Conformation Stability Which One Is Lower Energy Most books will show a chair conformation slightly sideways making it impossible to copy. These are ready to be exploited for drug design in the presence and also in the absence of detailed knowledge about the three-dimensional structure of the protein active site. Worse its really difficult to show which substituents are axial vs equatorial. Which of the following cyclohexanes could exist in a conformation with both methyls equatorial. To begin start by drawing two lines that are parallel to each other but not perfectly horizontal as shown here. Structure and conformations of decalins.
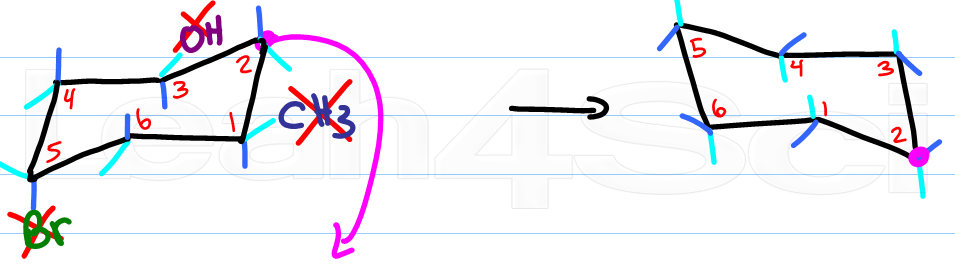 Source: leah4sci.com
Source: leah4sci.com
Drawing Chair Conformations And Ring Flips For Cyclohexane In Organic Chemistry Worse its really difficult to show which substituents are axial vs equatorial. The A-value of a tert-Butyl is in the range of 5 kcalmol which is around the value of the energy difference between cyclohexane in twist-boat and chair conformation. Finally add an upward-pointing V tip to the other end this is the nose of the chair. The chair is by far the most stable and only the skew conformation has an energy minimum in a similar range but this is still some 20 kJ higher than the chair. Driven by the wealth and diversity of bond angle and torsion information in the Cambridge Structural Database the CSD Conformer Generator produces realistic ensembles of low energy ligand structures. Structure and conformations of decalins.
 Source: chemistrysteps.com
Source: chemistrysteps.com
Ring Flip Of Chair Conformations With Practice Problems Chemistry Steps Driven by the wealth and diversity of bond angle and torsion information in the Cambridge Structural Database the CSD Conformer Generator produces realistic ensembles of low energy ligand structures. Rotate the molecule in the JSMOL image to show this just like a Newman projection so. Cyclic alkanes can also interconvert between their conformers by rotation of the single carbon to carbon bonds. Explaining how A-Values are related to cyclohexane flip energy. And now the stabilities. In a chair conformation with a few exceptions only axial substituents will give rise to steric strain energy.
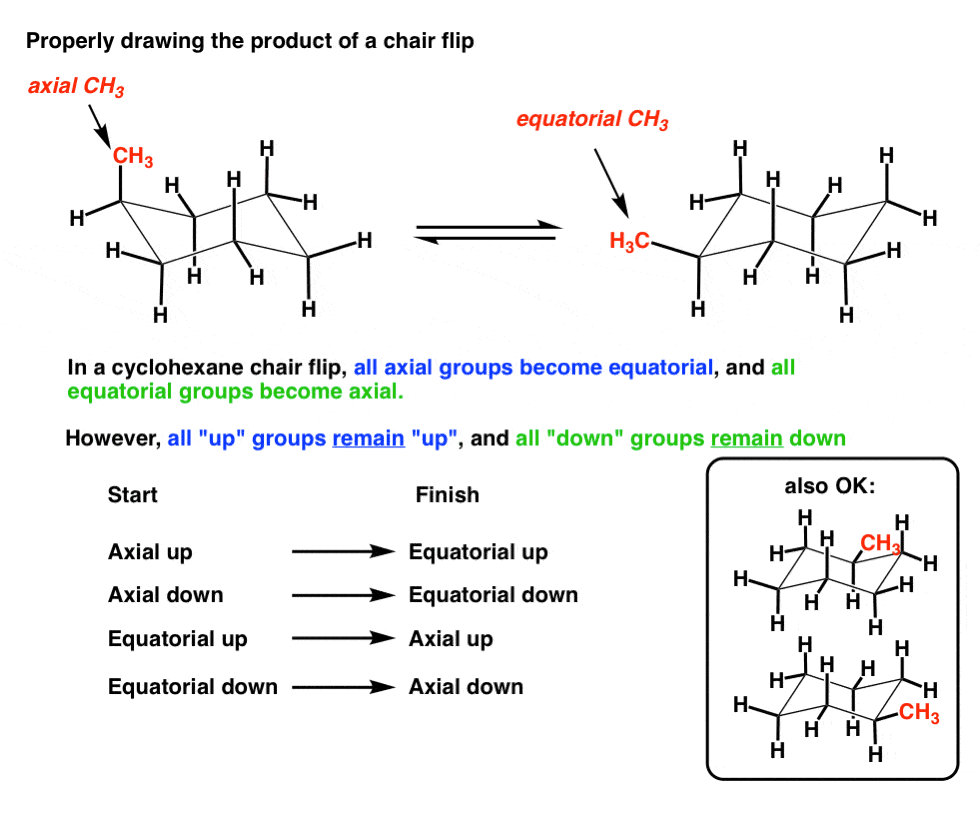 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
The Cyclohexane Chair Flip Master Organic Chemistry Explaining how A-Values are related to cyclohexane flip energy. Worse its really difficult to show which substituents are axial vs equatorial. First we have to introduce the concept of an A-value which is simply the energy difference between the equatorial most stable and axial least stable positions. Draw in the hydrogens and bromines on the left hand picture below. At first we need to change the hydroxyl group on C-5 in the proper orientation. The A-value of a tert-Butyl is in the range of 5 kcalmol which is around the value of the energy difference between cyclohexane in twist-boat and chair conformation.
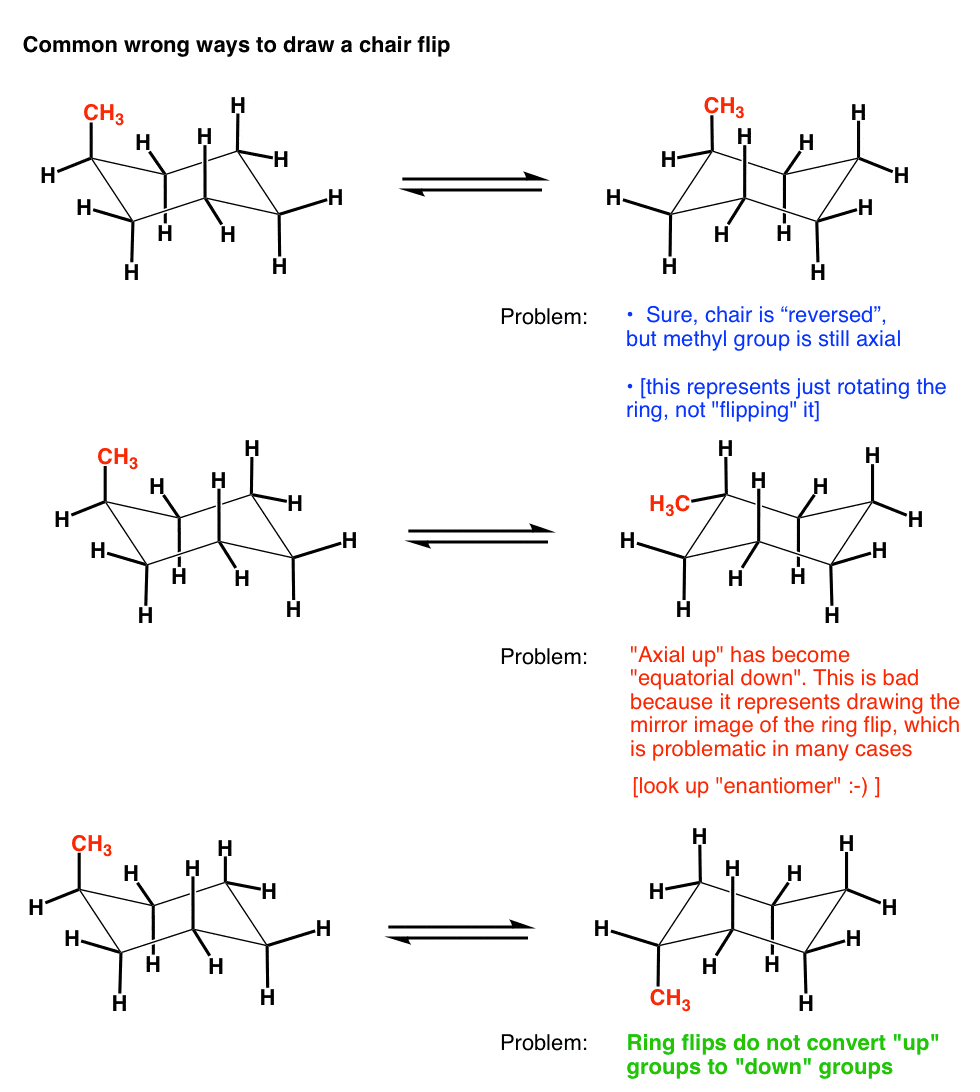 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
The Cyclohexane Chair Flip Master Organic Chemistry Fischer projection for the open-chain molecules Haworth projections focusing on cyclic pyranoses and your regular chair conformations. Explaining how A-Values are related to cyclohexane flip energy. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. Drawing a Sugars Chair Conformation from a Haworth Structure or Fischer Projection If you have either the Haworth structure or the Fischer projection of the sugar drawing the chair conformation of the sugar is easy as long as you remember the orientations of the axial and equatorial bonds on each carbon atom of the chair conformation. The steps involved in drawing the chair conformation of cyclohexane. In each of the conformations drawn in step 4 circle the axial substituents other than hydrogen.
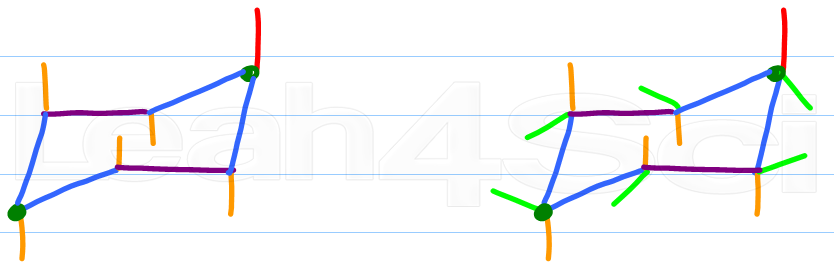 Source: leah4sci.com
Source: leah4sci.com
Drawing Chair Conformations And Ring Flips For Cyclohexane In Organic Chemistry Most books will show a chair conformation slightly sideways making it impossible to copy. Explaining how A-Values are related to cyclohexane flip energy. Organic Chemistry Carbohydrates Converting Between Fischer Haworth and Chair Forms of Carbohydrates In this post I want to go over the three most typical forms of the carbohydrates. First we have to introduce the concept of an A-value which is simply the energy difference between the equatorial most stable and axial least stable positions. Worse its really difficult to show which substituents are axial vs equatorial. Structure and conformations of decalins.
 Source: youtube.com
Source: youtube.com
Chair Conformation And Ring Flips Youtube First we have to introduce the concept of an A-value which is simply the energy difference between the equatorial most stable and axial least stable positions. Calculating Flip Energy. This is where the messiness and confusion arises. In a chair conformation with a few exceptions only axial substituents will give rise to steric strain energy. Draw The Correct Structure For Cis 1 3 Dichlorocyclopentane Show. While other structures are possible and youre definitely going to.
 Source: researchgate.net
Source: researchgate.net
Scheme 1 Fisher Projection Of D Xylose Centre And Haworth Projections Download Scientific Diagram Test your understanding with this Chair Conformations of Cyclohexane Quiz as part of the Cyclohexane Chair Conformation Video Tutorial Series. In a chair conformation with a few exceptions only axial substituents will give rise to steric strain energy. This chair-chair interconversion that leads to the generation of two equivalent energy forms is known as ring flipping. 1 3 Dibromocyclohexane Chair Conformation. Draw The Correct Structure For Cis 1 3 Dichlorocyclopentane Show. For 1 3 disubstituted cyclohexanes the cis form is diequatorial and the flipped conformation suffers additional steric interaction between the two axial groups.
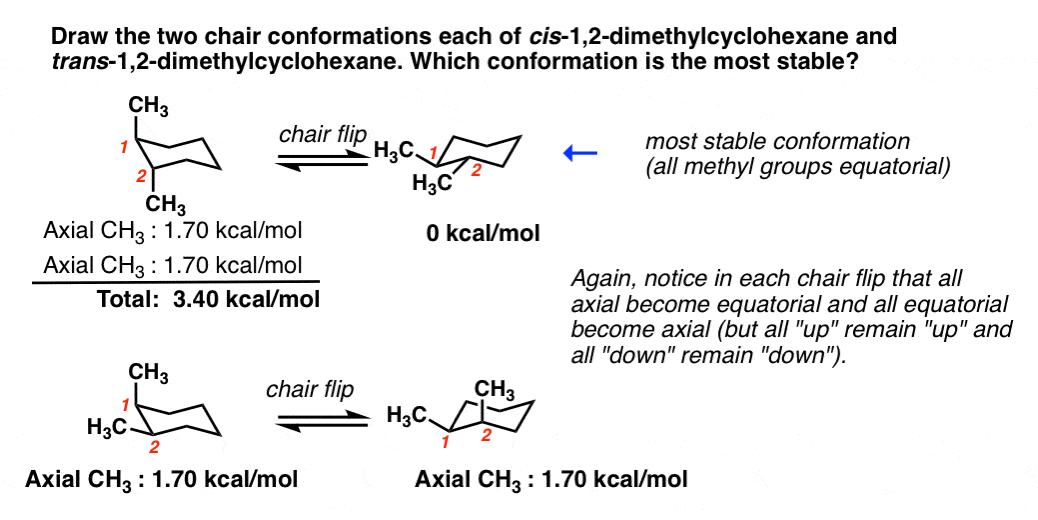 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Cyclohexane Chair Conformation Stability Which One Is Lower Energy Drawing a Sugars Chair Conformation from a Haworth Structure or Fischer Projection If you have either the Haworth structure or the Fischer projection of the sugar drawing the chair conformation of the sugar is easy as long as you remember the orientations of the axial and equatorial bonds on each carbon atom of the chair conformation. First we have to introduce the concept of an A-value which is simply the energy difference between the equatorial most stable and axial least stable positions. Organic Chemistry Carbohydrates Converting Between Fischer Haworth and Chair Forms of Carbohydrates In this post I want to go over the three most typical forms of the carbohydrates. To draw the chair conformation of D-glucose you need to know the orientations of the axial and equatorial bonds on carbon atoms of the chair conformation. This is true for 1R-33-dichlorocyclohexanol. While other structures are possible and youre definitely going to.
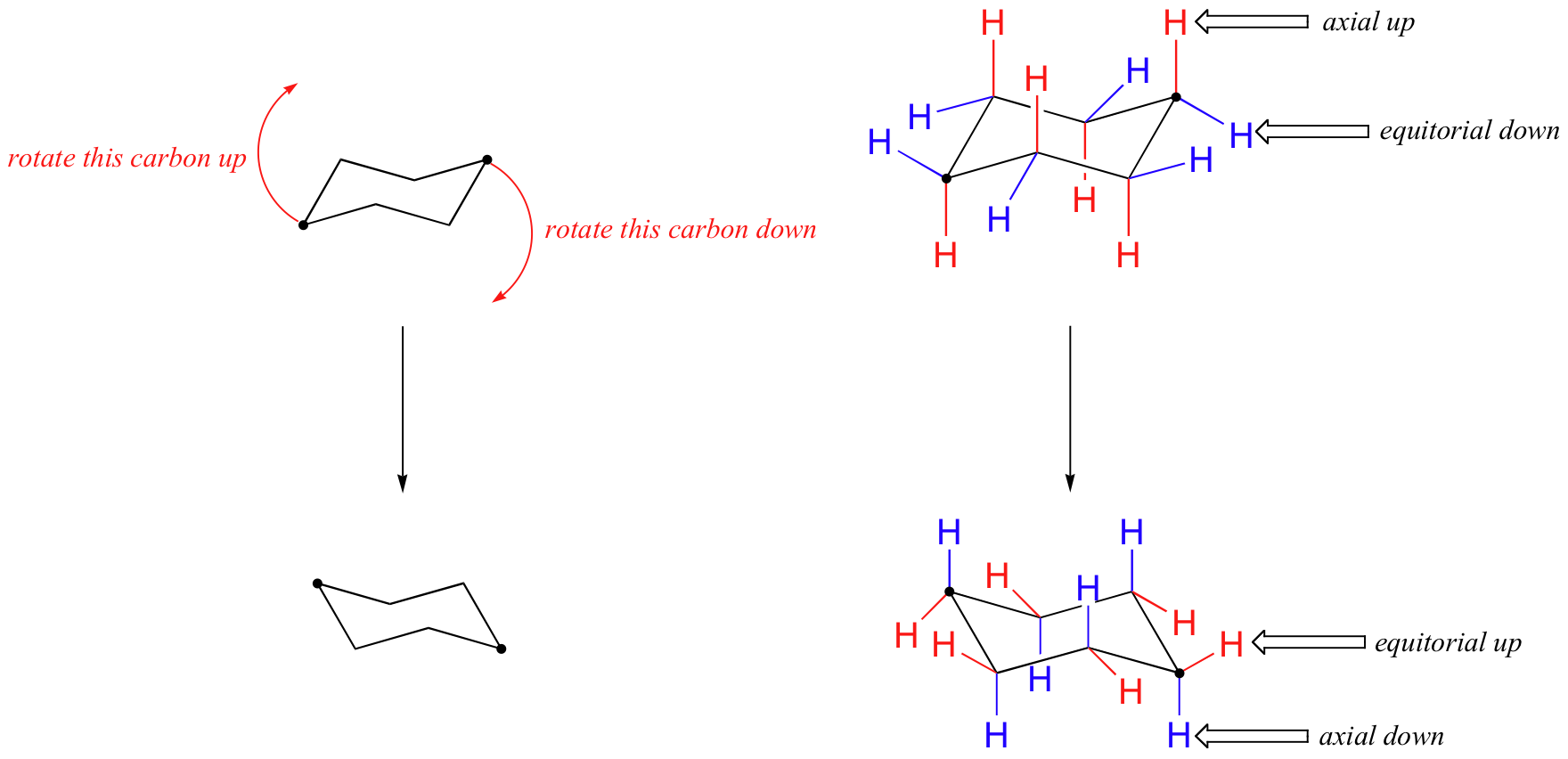 Source: chem.libretexts.org
Source: chem.libretexts.org
4 7 Cyclohexane Conformations Chemistry Libretexts The chair is by far the most stable and only the skew conformation has an energy minimum in a similar range but this is still some 20 kJ higher than the chair. In one conformation both methyl groups are axial in the other conformation both methyl groups are equatorialThese two conformers are not equivalent and the di-equatorial one is the more stable conformation as we would expect. This is true for 1R-33-dichlorocyclohexanol. Rotate the molecule in the JSMOL image to show this just like a Newman projection so. In the first conformer we have two chlorines in axial positions so the total steric strain is. However this is the best orientation for the chair conformation of 1R 2S 4R 24-dimethyl-1-tertbutylcyclohexane.
 Source: chemistrysteps.com
Source: chemistrysteps.com
Ring Flip Of Chair Conformations With Practice Problems Chemistry Steps Ive drawn cis-14-di-tert-butyl-cyclohexane with ChemDraw and used EditGet 3D Model and ChemBioDraw gave me the structure which is NOT the energetical most suitable one. Structure and conformations of decalins. Organic Chemistry Carbohydrates Converting Between Fischer Haworth and Chair Forms of Carbohydrates In this post I want to go over the three most typical forms of the carbohydrates. The key difference between chair and boat conformation is that chair conformation has low energy whereas boat conformation has high energy. For 1 3 disubstituted cyclohexanes the cis form is diequatorial and the flipped conformation suffers additional steric interaction between the two axial groups. At first we need to change the hydroxyl group on C-5 in the proper orientation.
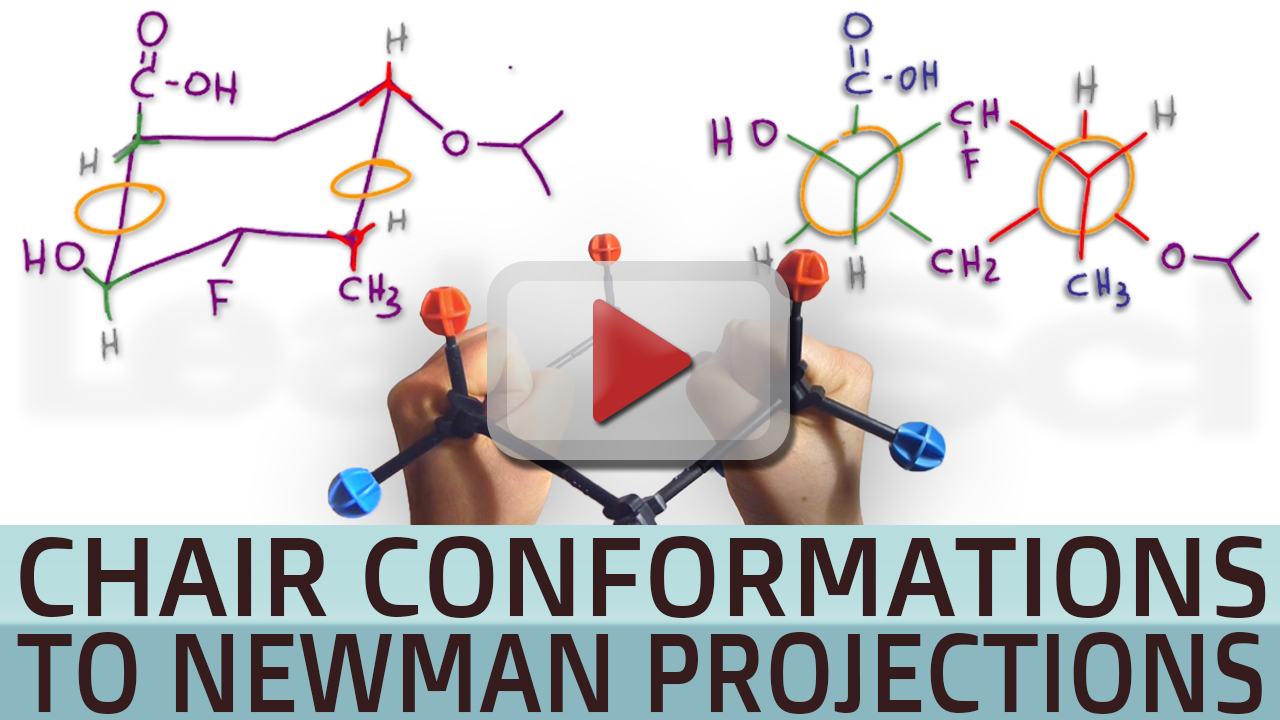 Source: leah4sci.com
Source: leah4sci.com
Cyclohexane Chair To Double Newman Quiz Challenge Question Mcat And Organic Chemistry Study Guides Tutoring For 1 3 disubstituted cyclohexanes the cis form is diequatorial and the flipped conformation suffers additional steric interaction between the two axial groups. In the first conformer we have two chlorines in axial positions so the total steric strain is. Drawing a Sugars Chair Conformation from a Haworth Structure or Fischer Projection If you have either the Haworth structure or the Fischer projection of the sugar drawing the chair conformation of the sugar is easy as long as you remember the orientations of the axial and equatorial bonds on each carbon atom of the chair conformation. Put all the groups in the equatorial position to generate the most stable chair conformation. This chair-chair interconversion that leads to the generation of two equivalent energy forms is known as ring flipping. Click the structures and reaction arrows to view the 3D models and animations respectively.
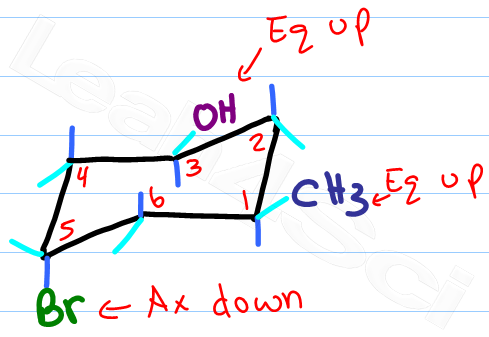 Source: leah4sci.com
Source: leah4sci.com
Drawing Chair Conformations And Ring Flips For Cyclohexane In Organic Chemistry First we have to introduce the concept of an A-value which is simply the energy difference between the equatorial most stable and axial least stable positions. However this is the best orientation for the chair conformation of 1R 2S 4R 24-dimethyl-1-tertbutylcyclohexane. The key difference between chair and boat conformation is that chair conformation has low energy whereas boat conformation has high energy. If you switch out a carbon atom for an oxygen atom and you are set to study the pyranoses of biochemistry eg. To draw the chair conformation of D-glucose you need to know the orientations of the axial and equatorial bonds on carbon atoms of the chair conformation. To begin start by drawing two lines that are parallel to each other but not perfectly horizontal as shown here.
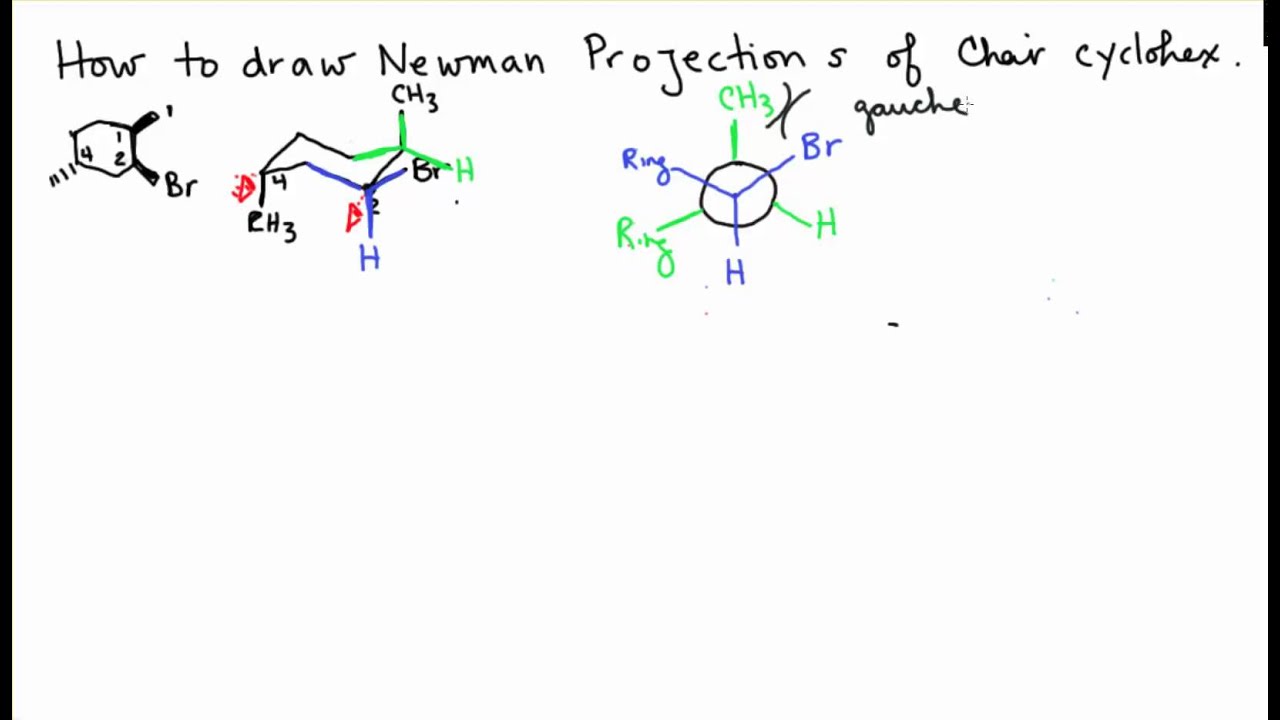 Source: youtube.com
Source: youtube.com
Double Newman Projection Chair Cyclohexane Youtube A chair conformation of cyclohexane can undergo a conformational change into another chair conformer by the partial rotation of C-C bonds. The C-C-C bonds are very close to 1095 o so it is almost free of angle strain. In one conformation both methyl groups are axial in the other conformation both methyl groups are equatorialThese two conformers are not equivalent and the di-equatorial one is the more stable conformation as we would expect. Draw the second chair conformation ring-flip-check this post if not sure. In a chair conformation with a few exceptions only axial substituents will give rise to steric strain energy. 1 3 Dibromocyclohexane Chair Conformation.
 Source: leah4sci.com
Source: leah4sci.com
Drawing Chair Conformations And Ring Flips For Cyclohexane In Organic Chemistry Converting Cyclohexane Chair to Double Newman Projection Tutorial Video. Explaining how A-Values are related to cyclohexane flip energy. For 1 3 disubstituted cyclohexanes the cis form is diequatorial and the flipped conformation suffers additional steric interaction between the two axial groups. Calculating Flip Energy. Ive drawn cis-14-di-tert-butyl-cyclohexane with ChemDraw and used EditGet 3D Model and ChemBioDraw gave me the structure which is NOT the energetical most suitable one. Explaining how A-Values are related to cyclohexane flip energy.
Please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark or be able to bookmark this blog page in this website. Thank you …










