Chair conformation highest energy The tert-butyl group needs to be placed in the equatorial bond as it is the lowest energy or highest stability conformation. B The methyl group occupies an equatorial position.
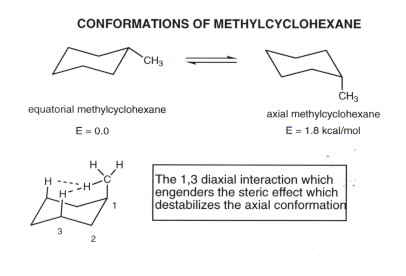
Chair Conformation Highest Energy, So the equatorial conformation is more stable than the axial by 728 kJmol. B The methyl group occupies an equatorial position. FIGURE 4-29 The two chair conformations of methylcyclohexane The two chair conformations of monosubstituted cyclohexanes are not equivalent.
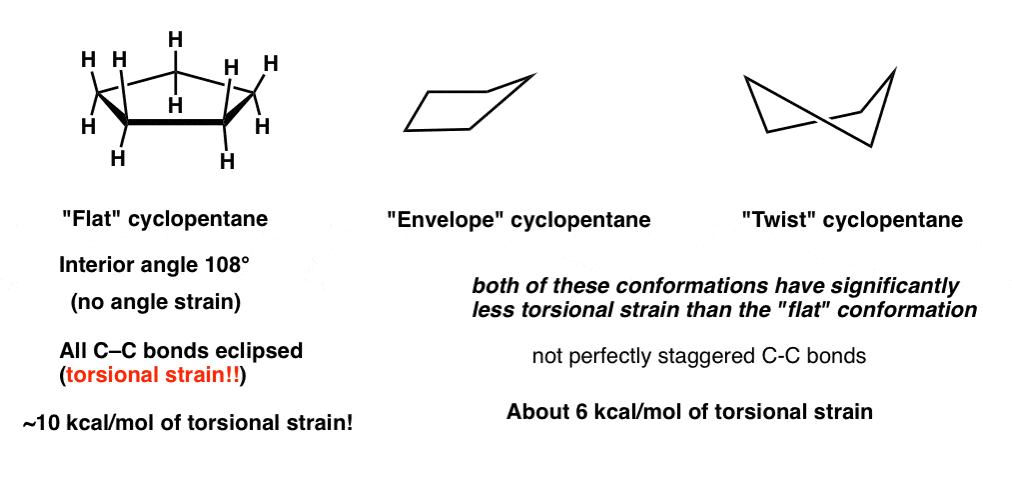 Cyclohexane Conformations Master Organic Chemistry From masterorganicchemistry.com
Cyclohexane Conformations Master Organic Chemistry From masterorganicchemistry.com
Another Article :
D The higher energy chair conformation contains two axial methyl groups. The tert-butyl group needs to be placed in the equatorial bond as it is the lowest energy or highest stability conformation. He identified boatchair chairboat and chairchair forms as high-energy conformations. However since this conformation is 55 kcalmol higher in energy than the chair conformation it is not a highly populated stateAgain most of the strain energy results from bonds which are eclipsed or partially eclipsed ie from torsional strain. Twisting of each gives the corresponding low-energy conformations of cyclononane Fig.
What is the most important result of ring inversion.
The highest energy conformation of cyclohexane is called the. This is a multistep process so here Im going to walk you through it from scratch. For most classes all you will need to know how to do is use equatorial preference to predict the most stable chair conformation. Any substituent that is axial in the original chair conformation becomes equatorial in the ring-inverted form and vice versa. However since this conformation is 55 kcalmol higher in energy than the chair conformation it is not a highly populated stateAgain most of the strain energy results from bonds which are eclipsed or partially eclipsed ie from torsional strain.
 Source: slidetodoc.com
Source: slidetodoc.com
Day 2 Quiz Questions Draw The Lewis Structure D The higher energy chair conformation contains two axial methyl groups. This is a multistep process so here Im going to walk you through it from scratch. So the equatorial conformation is more stable than the axial by 728 kJmol. Although it is more stable the chair conformation is much more rigid than the boat conformation. A The methyl group occupies an axial position. Draw the highest energy chair conformation for trans-1-chloro-3-methylcyclohexane.
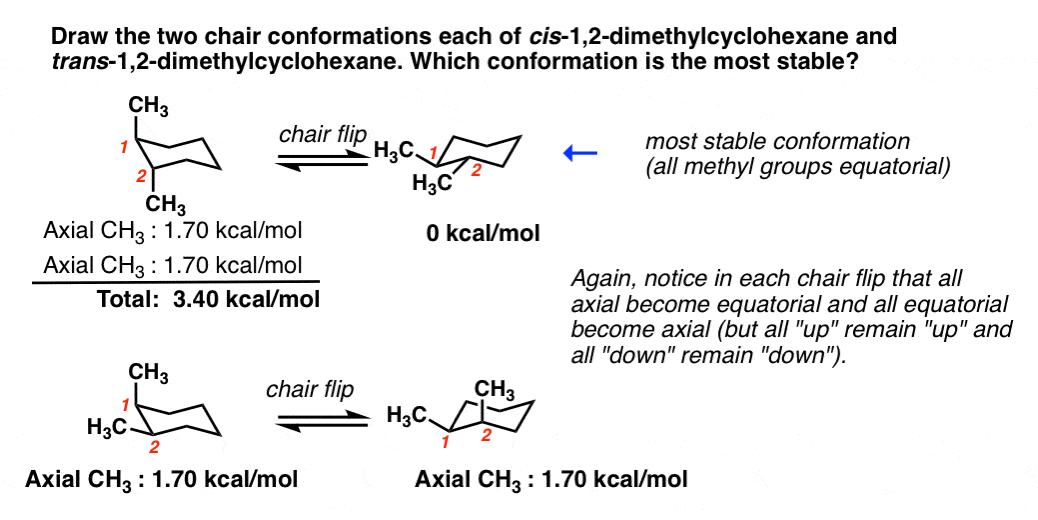 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Cyclohexane Chair Conformation Stability Which One Is Lower Energy FIGURE 4-29 The two chair conformations of methylcyclohexane The two chair conformations of monosubstituted cyclohexanes are not equivalent. This is a multistep process so here Im going to walk you through it from scratch. In the first conformer we have two chlorines in axial positions so the total steric strain is. This energy difference is known as the A value and it varies depending on the axial group. Justify why the half-chair conformation is so high in energy. B The methyl group occupies an equatorial position.
 Source: teachthemechanism.com
Source: teachthemechanism.com
Rules Of Thumb Rots For Chair Conformations And Substituent Stability Teach The Mechanism The tert-butyl group needs to be placed in the equatorial bond as it is the lowest energy or highest stability conformation. This is a multistep process so here Im going to walk you through it from scratch. The tert-butyl group needs to be placed in the equatorial bond as it is the lowest energy or highest stability conformation. Although it is more stable the chair conformation is much more rigid than the boat conformation. The larger the group the higher the energy difference. The key difference between chair and boat conformation is that a chair conformation has low energy whereas a boat conformation has high energy.
 Source: teachthemechanism.com
Source: teachthemechanism.com
Rules Of Thumb Rots For Chair Conformations And Substituent Stability Teach The Mechanism B The higher energy chair conformation contains one axial methyl group and one equatorial methyl group. However sometimes you will be required to use energetics to calculate the exact percentages of each chair in solution. Estimate the energy difference between the two. D The higher energy chair conformation contains two axial methyl groups. The lowest energy conformation is the chair conformation. B The higher energy chair conformation contains one axial methyl group and one equatorial methyl group.
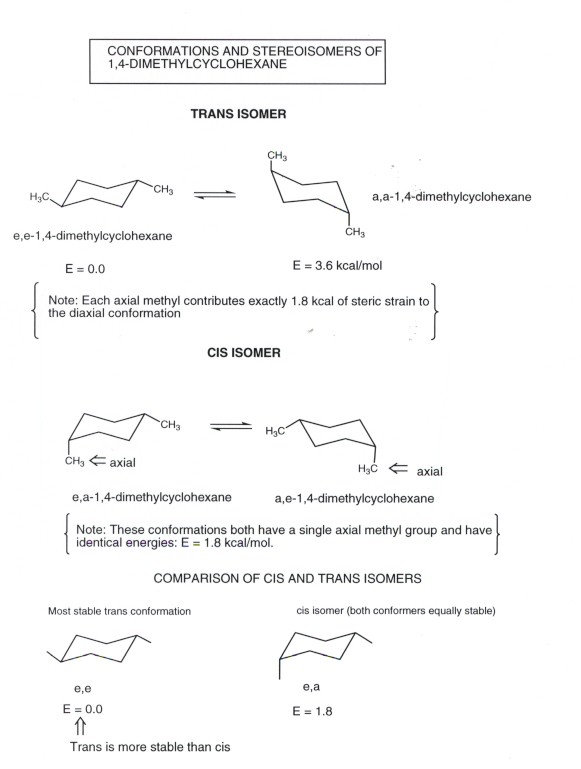 Source: research.cm.utexas.edu
Source: research.cm.utexas.edu
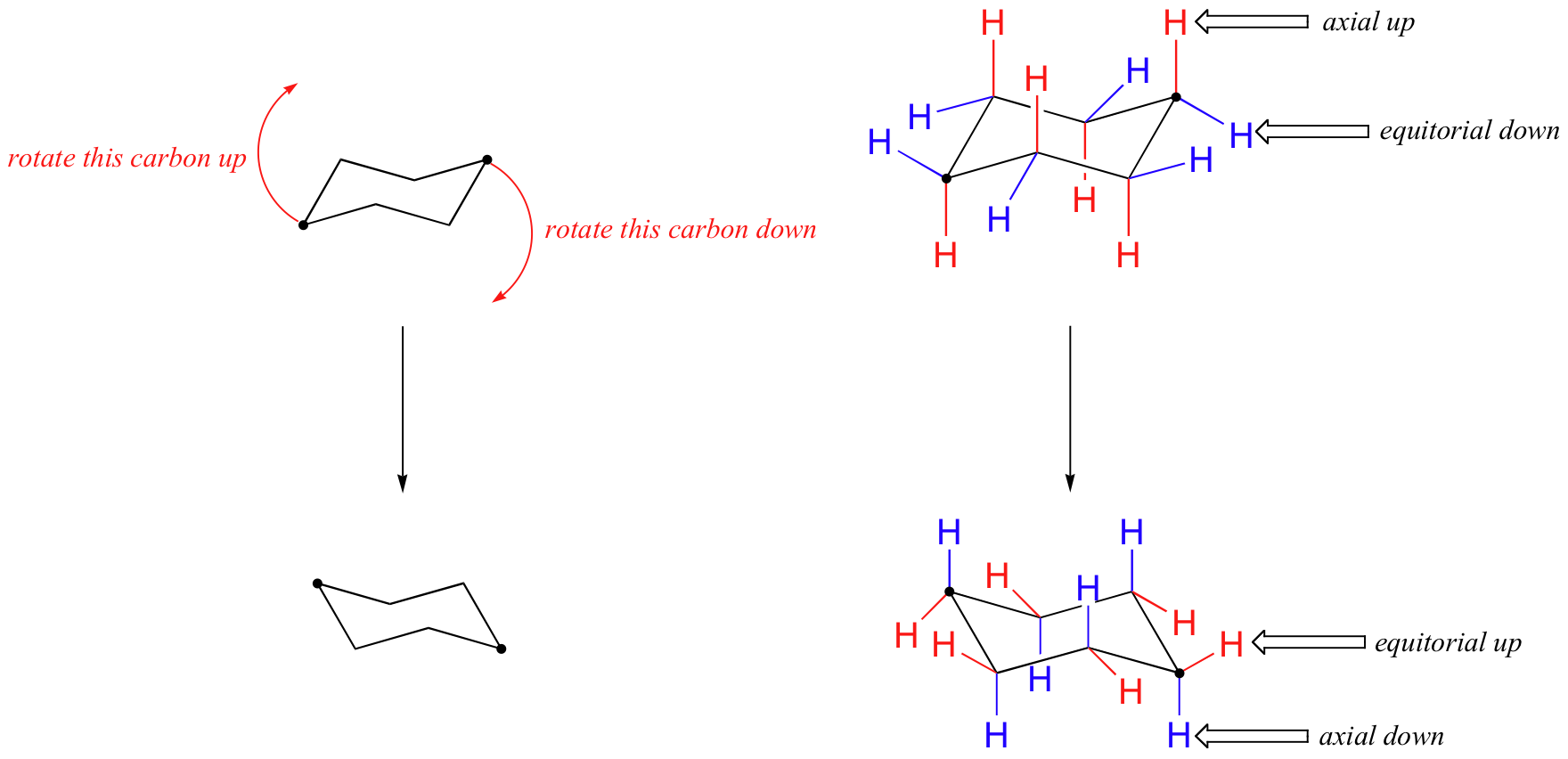
Cyclohexane Conformational Analysis For these reasons the boat conformation is a high energy conformation of cyclohexane about 30 kJmol less stable than the chair conformation. This is a multistep process so here Im going to walk you through it from scratch. Twist-boat and boat conformations are higher in energy than chair and are similar in energy levels although. Estimate the energy difference between the two. Boat chair The boat and chair conformations are interconvertable by passing through some very unstable high energy structures called the half-chair and the twist-boat. If you look carefully at your model of cyclohexane in the chair conformation you will see that all twelve hydrogens are not equivalent in terms of their three-dimensional arrangement in space.
 Source: chem.libretexts.org
Source: chem.libretexts.org
4 7 Cyclohexane Conformations Chemistry Libretexts The larger the group the higher the energy difference. An unstable conformation of cyclohexane is the boat conformation which is 71 kcalmol higher in energy than the chair form. Twist-boat and boat conformations are higher in energy than chair and are similar in energy levels although. Although it is more stable the chair conformation is much more rigid than the boat conformation. Cyclohexane is the chair conformation shown below. Draw the highest energy chair conformation for trans-1-chloro-3-methylcyclohexane.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
Why Is This Chair Conformation The Most Stable And Which Leaving Group Is Best Chemistry Stack Exchange Draw 2 chair conformations for the compound 124-trimethylcyclohexane B. The terms chair conformation and boat conformation come under organic chemistry and they are mainly applicable to cyclohexaneThese are two different structures in which the cyclohexane molecule can exist but they have different stabilities. Chair twist twist twist twist boat Note. Thus it is the most popular. 2222 44 kJ. So the equatorial conformation is more stable than the axial by 728 kJmol.
 Source: chem.ucalgary.ca
Source: chem.ucalgary.ca
Ch 3 Cyclohexane B The methyl group occupies an equatorial position. So the equatorial conformation is more stable than the axial by 728 kJmol. This energy difference is known as the A value and it varies depending on the axial group. However keep in mind that the geometry of the cyclohexane needs to be. Although it is more stable the chair conformation is much more rigid than the boat conformation. Torsional strain and flagpole interactions cause the boat conformation to have considerably higher energy than the chair conformation.
 Source: research.cm.utexas.edu
Source: research.cm.utexas.edu
Cyclohexane Conformational Analysis For most classes all you will need to know how to do is use equatorial preference to predict the most stable chair conformation. The tert-butyl group needs to be placed in the equatorial bond as it is the lowest energy or highest stability conformation. This energy difference is known as the A value and it varies depending on the axial group. If the tert-butyl group is placed in the axial bond then the chair has the highest energy or the least stable conformation. Cyclohexane is the chair conformation shown below. So the equatorial conformation is more stable than the axial by 728 kJmol.
 Source: chegg.com
Source: chegg.com
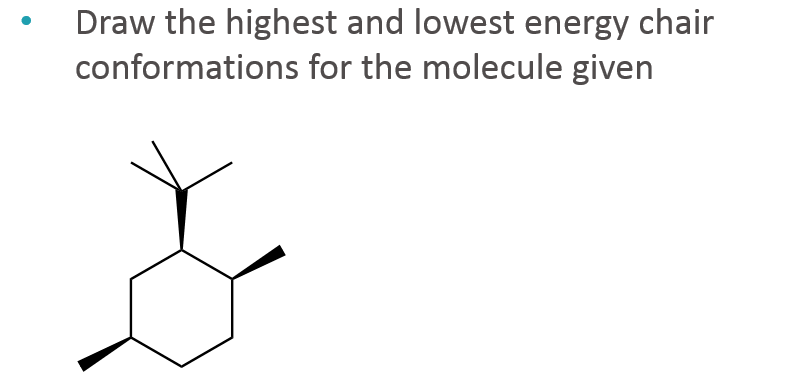
Solved Draw The Highest And Lowest Energy Chair Chegg Com For these reasons the boat conformation is a high energy conformation of cyclohexane about 30 kJmol less stable than the chair conformation. The highest energy conformation of cyclohexane is called the. Although it is more stable the chair conformation is much more rigid than the boat conformation. However since this conformation is 55 kcalmol higher in energy than the chair conformation it is not a highly populated stateAgain most of the strain energy results from bonds which are eclipsed or partially eclipsed ie from torsional strain. The key difference between chair and boat conformation is that a chair conformation has low energy whereas a boat conformation has high energy. The lowest energy conformation is the chair conformation.

4 6 Axial And Equatorial Bonds In Cyclohexane Chemistry Libretexts For these reasons the boat conformation is a high energy conformation of cyclohexane about 30 kJmol less stable than the chair conformation. The structures you generate may differ from those seen below. Draw the highest energy chair conformation for trans-1-chloro-3-methylcyclohexane. Justify why the half-chair conformation is so high in energy. This is a multistep process so here Im going to walk you through it from scratch. The larger the group the higher the energy difference.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
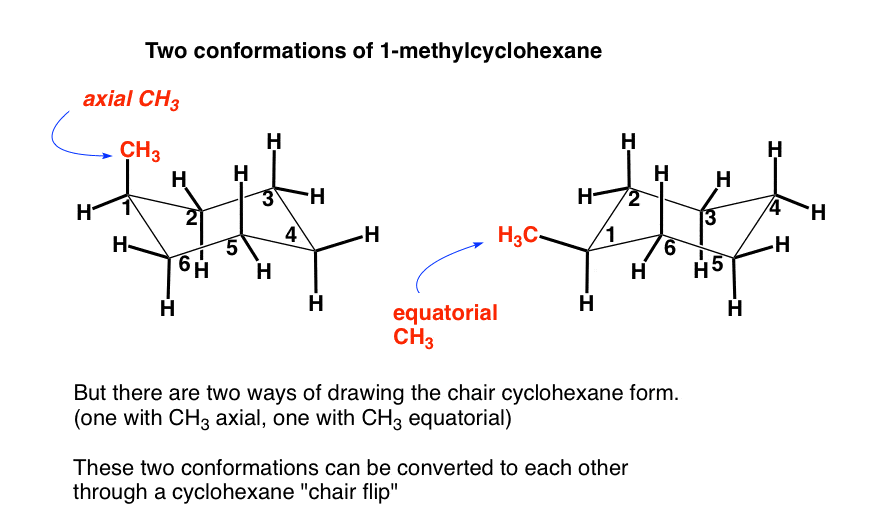
The Cyclohexane Chair Flip Master Organic Chemistry Draw the highest energy chair conformation for trans-1-chloro-3-methylcyclohexane. Draw the second chair conformation ring-flip-check this post if not sure. D The higher energy chair conformation contains two axial methyl groups. However since this conformation is 55 kcalmol higher in energy than the chair conformation it is not a highly populated stateAgain most of the strain energy results from bonds which are eclipsed or partially eclipsed ie from torsional strain. Although it is more stable the chair conformation is much more rigid than the boat conformation. For example the energy difference of the axial ethyl cyclohexane with the equatorial conformer is 73 kJmol.
 Source: research.cm.utexas.edu
Source: research.cm.utexas.edu
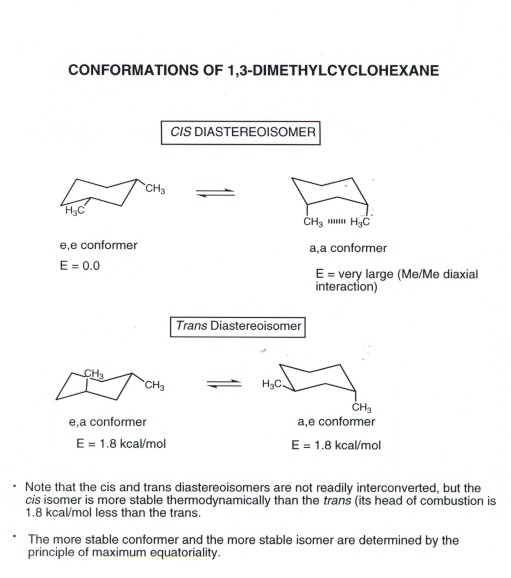
Cyclohexane Conformational Analysis B The methyl group occupies an equatorial position. If the tert-butyl group is placed in the axial bond then the chair has the highest energy or the least stable conformation. Draw the highest energy chair conformation for trans-1-chloro-3-methylcyclohexane. An unstable conformation of cyclohexane is the boat conformation which is 71 kcalmol higher in energy than the chair form. FIGURE 4-29 The two chair conformations of methylcyclohexane The two chair conformations of monosubstituted cyclohexanes are not equivalent. For each chair conformer add the energy of all the groups on axial position.
 Source: research.cm.utexas.edu
Source: research.cm.utexas.edu
Cyclohexane Conformational Analysis And now the stabilities. Draw the following in the lowest energy chair conformation. At ОН Question 3 Draw resonance structures for the following compounds and label the major contributor NH2 H2N Question 4 Convert the following. Draw the highest energy chair conformation for trans-1-chloro-3-methylcyclohexane. Therefore the chair conformation is more stable than boat conformation at room temperature. A The methyl group occupies an axial position.
 Source: chegg.com
Source: chegg.com
Solved A Which Has The Highest Energy Diaxial Chair Chegg Com He identified boatchair chairboat and chairchair forms as high-energy conformations. If the tert-butyl group is placed in the axial bond then the chair has the highest energy or the least stable conformation. What is the most important result of ring inversion. E The lower energy chair conformation contains two axial methyl groups. Justify why the half-chair conformation is so high in energy. Thus it is the most popular.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Cyclohexane Chair Conformation Stability Which One Is Lower Energy The highest energy conformation of cyclohexane is called the. However sometimes you will be required to use energetics to calculate the exact percentages of each chair in solution. The structures you generate may differ from those seen below. 2222 44 kJ. For most classes all you will need to know how to do is use equatorial preference to predict the most stable chair conformation. Not a potential energy maximum but a local minimum on the potential energy profile.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
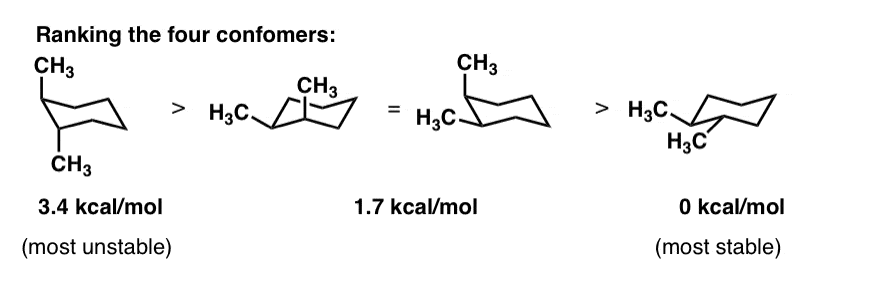
Cyclohexane Conformations Master Organic Chemistry 2222 44 kJ. The structures you generate may differ from those seen below. So the equatorial conformation is more stable than the axial by 728 kJmol. Cyclohexane is the chair conformation shown below. For these reasons the boat conformation is a high energy conformation of cyclohexane about 30 kJmol less stable than the chair conformation. Twisting of each gives the corresponding low-energy conformations of cyclononane Fig.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Cyclohexane Chair Conformation Stability Which One Is Lower Energy In the first conformer we have two chlorines in axial positions so the total steric strain is. Calculations show that a twist-boatchair TBC is the lowest energy form. B The methyl group occupies an equatorial position. However since this conformation is 55 kcalmol higher in energy than the chair conformation it is not a highly populated stateAgain most of the strain energy results from bonds which are eclipsed or partially eclipsed ie from torsional strain. The highest energy conformation of cyclohexane is called the. C The lower energy chair conformation contains one axial methyl group and one equatorial methyl group.
Please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark or be able to bookmark this blog page in this website. Thank you …










