Chair conformation methylcyclohexane Select both chair conformations of methylcyclohexane. Cyclohexane and the Chair Structure.
Chair Conformation Methylcyclohexane, Before deciding whether or not this is the most stable chair conformation you must draw the chair flip conformation as well. As you can see this drawing shows them both on wedges. Select both chair conformations of methylcyclohexane.
 Solved Hi I Have A Problem With Drawing Chair Conformations Chegg Com From chegg.com
Solved Hi I Have A Problem With Drawing Chair Conformations Chegg Com From chegg.com
Another Article :
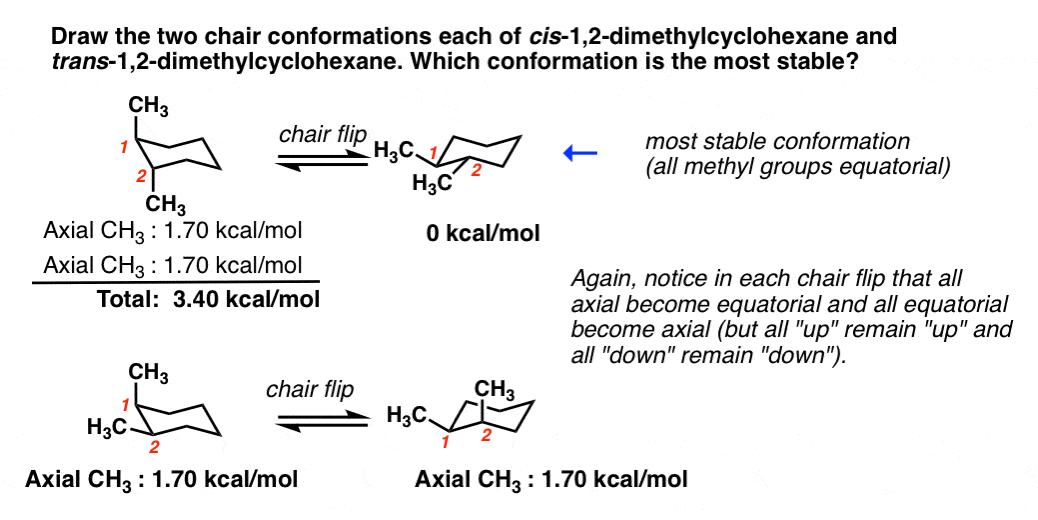
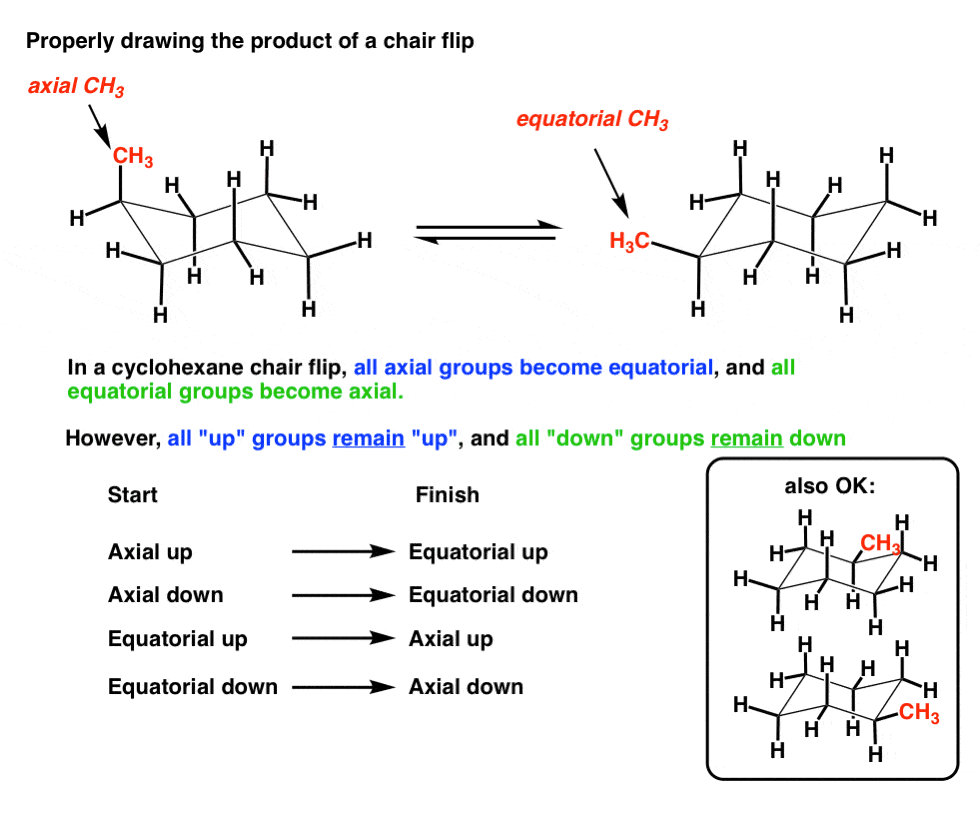
In each of the two boxes below draw in a bond to one methyl CH3 group in the appropriate position. Figure 76 The chair interconversion results in an equilibrium between equatorial left and axial right confor-mations of methylcyclohexaneThe conversion is shown with two different ring perspectivesNotice in this inter-conversion that a down methyl remains down and an up methyl remains up. 2 pt CH3 Least stable chair Most stable chair 1 1 1 Ð18 kcalmolG. As you can see this drawing shows them both on wedges. For Teachers for Schools for Working Scholars.
In this conformation there is no torsional strain at all and as we shall see later no strain of any kind.
Comparison of the different chair conformations of methylcyclohexane About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube. In conformation Fig3a the methyl group with yellow hydrogens in the space-filling model occupies an axial position and in conformation II the methyl group occupies an equatorial position. The tert-butyl group equatorial and the methyl group axial. Thus at 50C 938 of equatorial conformation and 62 of axial conformation exists in equilibrium. Before deciding whether or not this is the most stable chair conformation you must draw the chair flip conformation as well.
 Source: socratic.org
Source: socratic.org
What Are The Conformers Of Ethane And Methylcyclohexane Socratic The chair structure consists of a six-membered ring where every C-C bond exists in a staggered conformation. Comparison of the different chair conformations of methylcyclohexane About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube. At any instant almost all the methylcyclohexane molecules in a given sample exist in chair conformations and about 95. 1 What is the percentage contribution of the axial and equatorial conformations of methylcyclohexane at 25 50 100 and 150C. Methylcyclohexane has two possible chair conformations Fig3 I and II and these are interconvertible through the bond rotations that constitute a ring flip. Click hereto get an answer to your question Write the structures of two chair conformations of 1 - tert - butyl - 2 - methylcyclohexane.
 Source: research.cm.utexas.edu
Source: research.cm.utexas.edu
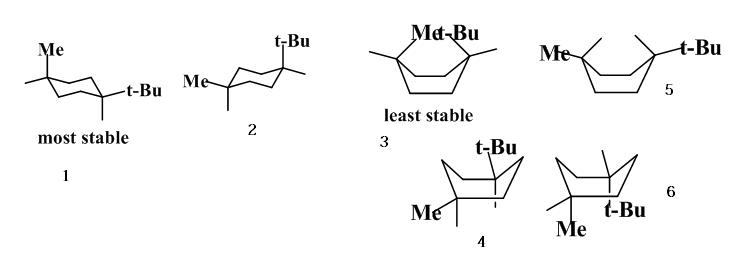
Cyclohexane Conformational Analysis Which conformation is more stable. Before deciding whether or not this is the most stable chair conformation you must draw the chair flip conformation as well. In the given figure various possible chair conformations of 12-dimethylcyclohexane are drawn. Which conformation is more stable. Thus at 50C 938 of equatorial conformation and 62 of axial conformation exists in equilibrium. The conformation with the bulky groups.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Cyclohexane Chair Conformation Stability Which One Is Lower Energy At 25 C 9999 of all molecules in a cyclohexane solution adopt this conformation. At 100C 913. The most stable conformation of methylcyclohexane is the chair conformation in which the methyl group is equatorial. With no torsional strain and no angle strain cyclohexane is the most stable of all the small rings of. The chair structure consists of a six-membered ring where every C-C bond exists in a staggered conformation. The above image shows both of them on wedges.
 Source: chegg.com
Source: chegg.com
Solved Hi I Have A Problem With Drawing Chair Conformations Chegg Com Who are the experts. Start by drawing the wedge-dash notation for cis-1-ethyl-2-methylcyclohexane which would look something like this. Thus at equilibrium and 25C 95 of equatorial-conformation and 5 of axial-conformation exists. With no torsional strain and no angle strain cyclohexane is the most stable of all the small rings of. As you can see this drawing shows them both on wedges. 2 pt CH3 Least stable chair Most stable chair 1 1 1 Ð18 kcalmolG.
 Source: kpu.pressbooks.pub
Source: kpu.pressbooks.pub
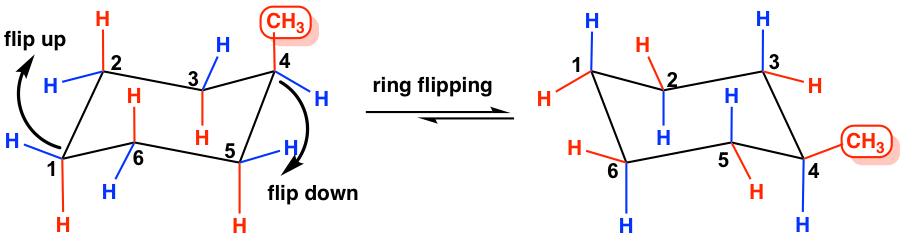
4 4 Substituted Cyclohexanes Organic Chemistry At any instant almost all the methylcyclohexane molecules in a given sample exist in chair conformations and about 95. The most stable conformation of methylcyclohexane is the chair conformation in which the methyl group is equatorial. Cyclohexane is unique in being the only cyclic hydrocarbon which is. This problem has been solved. The ground state conformation of cyclohexane is a fully staggered conformation which is shaped somewhat like a chair. Click hereto get an answer to your question Write the structures of two chair conformations of 1 - tert - butyl - 2 - methylcyclohexane.
 Source: chegg.com
Source: chegg.com
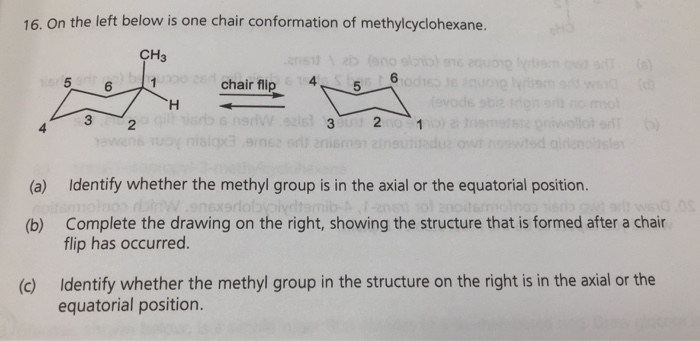
Solved 16 On The Left Below Is One Chair Conformation Of Chegg Com Draw the two-chair confirmation of trans 1-isopropyl -3-methylcyclohexane. The above image shows both of them on wedges. SInce the ethyl and methyl group are in a cis relationship both must be placed either on wedges or on dashes. Draw the two-chair confirmation of trans 1-isopropyl -3-methylcyclohexane. The most stable conformation of methylcyclohexane is the chair conformation in which the methyl group is equatorial. The most stable chair conformation of cis-1-tert-butyl-3-methylcyclohexane has.
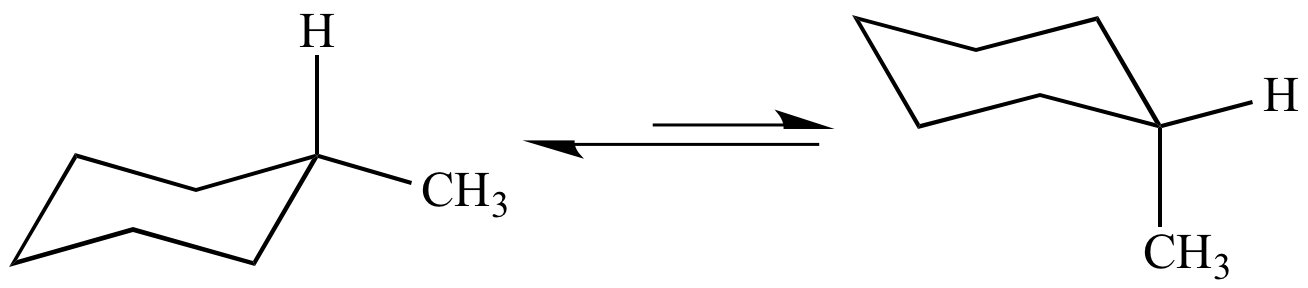 Source: chem.ucla.edu
Source: chem.ucla.edu
Illustrated Glossary Of Organic Chemistry Chair Conformation For Teachers for Schools for Working Scholars. The first chair conformation for the compound looks like this The chloro group is UP in axial position the same as the methyl group which is UP in axial. Choose a chair from the Templates toolbar at the bottom. 1 What is the percentage contribution of the axial and equatorial conformations of methylcyclohexane at 25 50 100 and 150C. In this conformation there is no torsional strain at all and as we shall see later no strain of any kind. Cyclohexane and the Chair Structure.
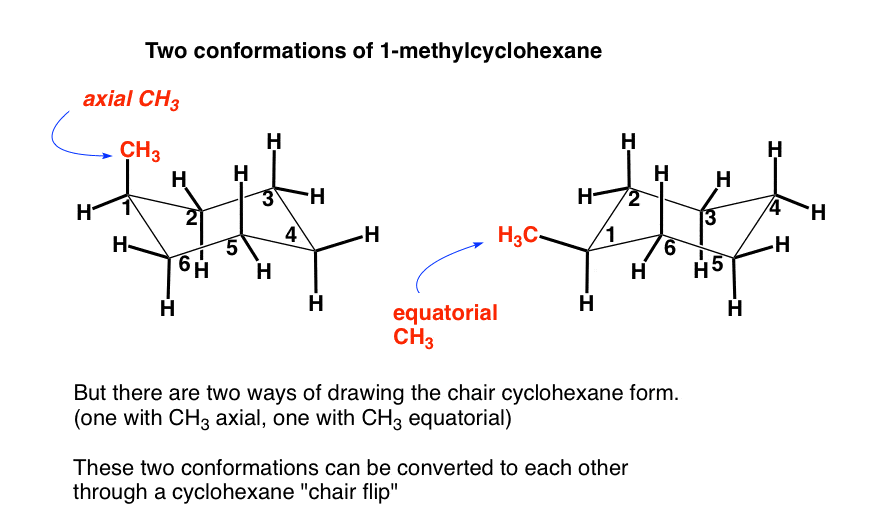 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
The Cyclohexane Chair Flip Master Organic Chemistry The alternative chair conformation in which the methyl group is axial is 73 kJmol higher in energy. Draw cis-1-ethyl-4-methylcyclohexane in its lowest energy conformation Part A Draw l-ethyl-4-methylcyclohexane in its lowest energy conformation 1. This problem has been solved. The first chair conformation for the compound looks like this The chloro group is UP in axial position the same as the methyl group which is UP in axial. In methylcyclohexane the chair conformation in which the large methyl group is equatorial is the most stable and therefore the most populated of all possible conformations. At any instant almost all the methylcyclohexane molecules in a given sample exist in chair conformations and about 95.
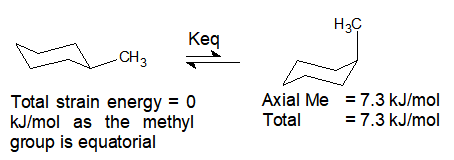 Source: organicchemistrytutorials.com
Source: organicchemistrytutorials.com
Blog 05 Calculate Percentage Of Chair Conformation In Cyclohexane Start by drawing an empty chair flip template. Start by drawing an empty chair flip template. The chair conformation is the most stable conformer. In the given figure various possible chair conformations of 12-dimethylcyclohexane are drawn. The chair structure of cyclohexane is considered to be the perfect conformation. There are two possibilities that are cis or trans but the position of the methyl group on axial or equatorial bond on cyclohexane determines whether the compound is cis or trans.
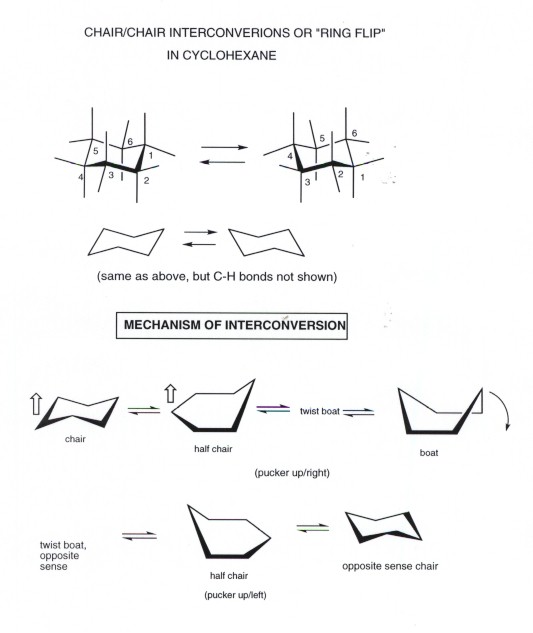 Source: research.cm.utexas.edu
Source: research.cm.utexas.edu
Cyclohexane Conformational Analysis Show transcribed image text Expert Answer. A Methylcyclohexane can exist in two different chair conformations one of which is 18 kcalmol more stable than the other ie the A value for the methyl group is 18. In this conformation there is no torsional strain at all and as we shall see later no strain of any kind. At any instant almost all the methylcyclohexane molecules in a given sample exist in chair conformations and about 95. Before deciding whether or not this is the most stable chair conformation you must draw the chair flip conformation as well. Start with the wedge-dash notation for cis-1-chloro-3-methylcyclohexane which looks like this Since the compound is cis the chloro and the methyl groups must either be on a wedge or on a dash.
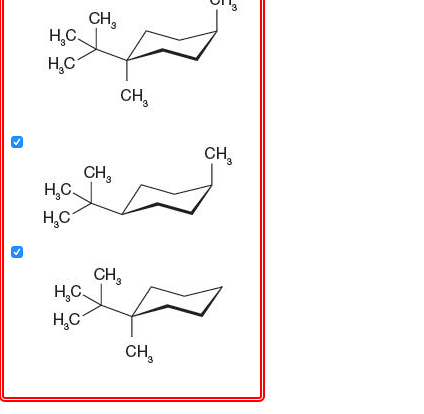 Source: chegg.com
Source: chegg.com
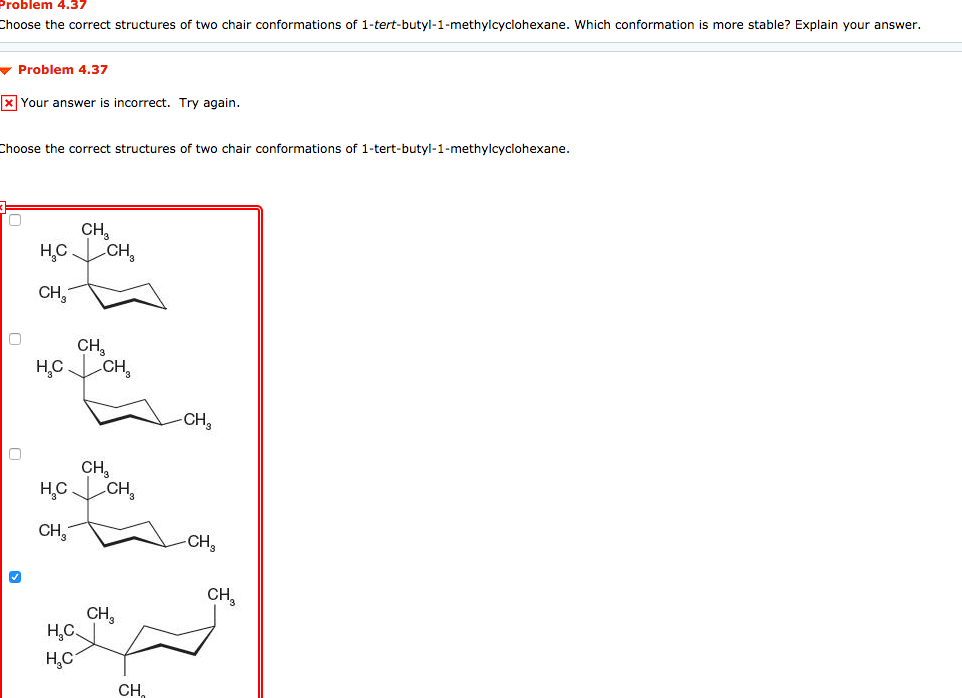
Solved Problem 4 37 Choose The Correct Structures Of Two Chegg Com The chair conformation is the most stable conformer. Draw the two-chair confirmation of trans 1-isopropyl -3-methylcyclohexane. In each of the two boxes below draw in a bond to one methyl CH3 group in the appropriate position. 2 pt CH3 Least stable chair Most stable chair 1 1 1 Ð18 kcalmolG. This difference corresponds to a equatorialaxial conformer ratio of. Start by drawing the wedge-dash notation for cis-1-ethyl-2-methylcyclohexane which would look something like this.

Answer In Organic Chemistry For Priskilla 92075 Methylcyclohexane has two possible chair conformations Fig3 I and II and these are interconvertible through the bond rotations that constitute a ring flip. The ground state conformation of cyclohexane is a fully staggered conformation which is shaped somewhat like a chair. Show transcribed image text Expert Answer. Choose a chair from the Templates toolbar at the bottom. Cyclohexane is unique in being the only cyclic hydrocarbon which is. For Teachers for Schools for Working Scholars.
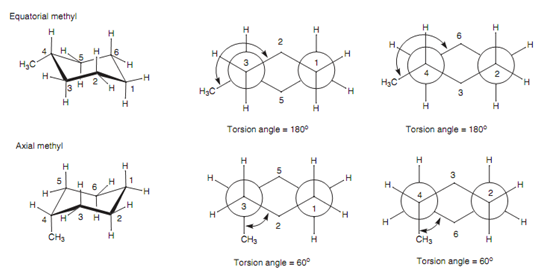 Source: expertsmind.com
Source: expertsmind.com
Newman Projections Of The Chair Conformations Of Methylcyclohexane Cycloalkanes Assignment Help Who are the experts. The chair form of the cyclohexane is most stable. The chair structure of cyclohexane is considered to be the perfect conformation. We review their content and use your feedback to keep the. Before deciding whether or not this is the most stable chair conformation you must draw the chair flip conformation as well. In each of the two boxes below draw in a bond to one methyl CH3 group in the appropriate position.
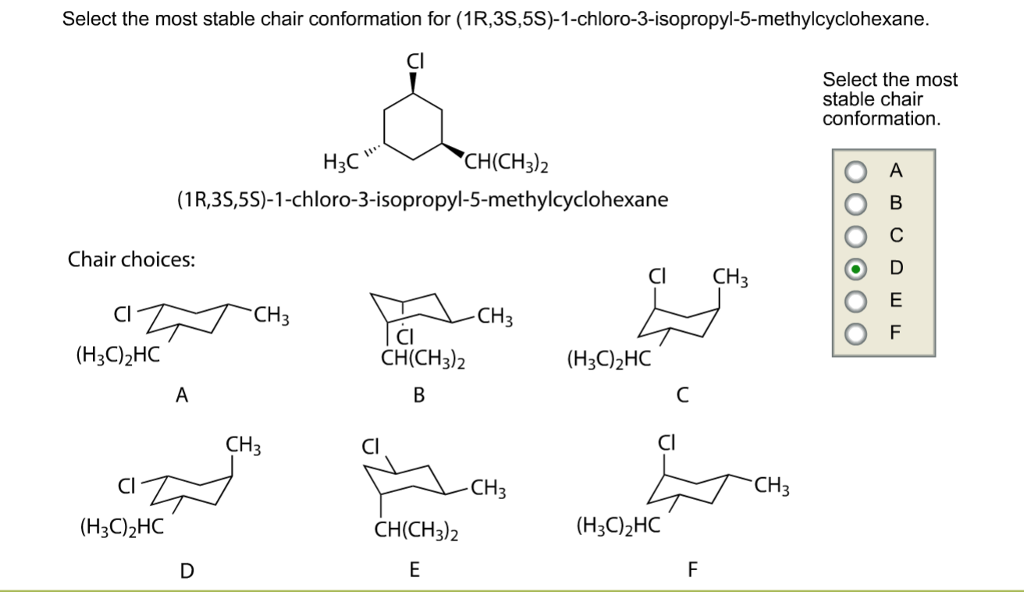 Source: chegg.com
Source: chegg.com
Solved Select The Most Stable Chair Conformation For Chegg Com Start with the wedge-dash notation for cis-1-chloro-3-methylcyclohexane which looks like this Since the compound is cis the chloro and the methyl groups must either be on a wedge or on a dash. A cyclohexane is a six-membered saturated cyclic ring with a chemical formula C6H12 C 6 H 12. The different conformations are called conformers a blend of the words conformation and isomer. The above image shows both of them on wedges. The ground state conformation of cyclohexane is a fully staggered conformation which is shaped somewhat like a chair. Figure 76 The chair interconversion results in an equilibrium between equatorial left and axial right confor-mations of methylcyclohexaneThe conversion is shown with two different ring perspectivesNotice in this inter-conversion that a down methyl remains down and an up methyl remains up.
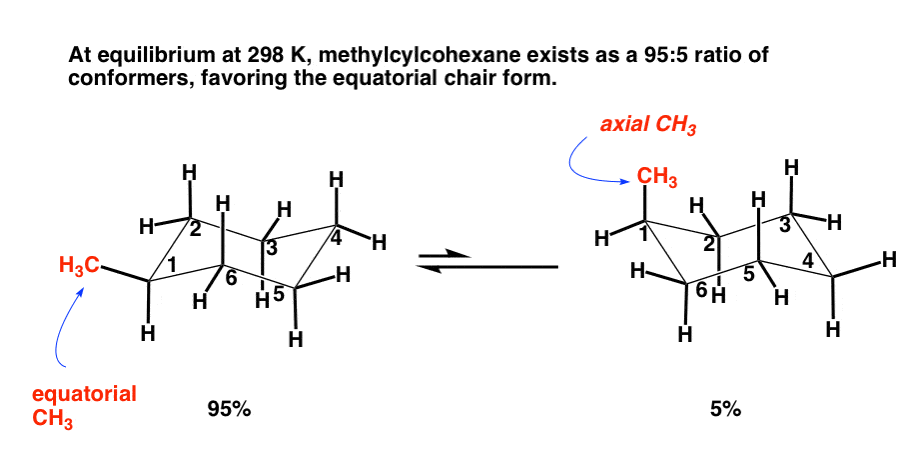 Source: brainstudy.info
Source: brainstudy.info
Methylcyclohexane Isomers Methylcyclohexane has two possible chair conformations Fig3 I and II and these are interconvertible through the bond rotations that constitute a ring flip. The chair conformation is the most stable conformer. The most stable conformation of methylcyclohexane is the chair conformation in which the methyl group is equatorial. H H C H H H H H C H H H a b c van der Waals repulsions. At 100C 913. For Teachers for Schools for Working Scholars.
 Source: chegg.com
Source: chegg.com
Solved Problem 4 37 Choose The Correct Structures Of Two Chegg Com Methylcyclohexane has two possible chair conformations Fig3 I and II and these are interconvertible through the bond rotations that constitute a ring flip. In this conformation there is no torsional strain at all and as we shall see later no strain of any kind. Select both chair conformations of methylcyclohexane. Choose a chair from the Templates toolbar at the bottom. Start by drawing an empty chair flip template. For Teachers for Schools for Working Scholars.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
The Cyclohexane Chair Flip Master Organic Chemistry Which conformation is more stable. At any instant almost all the methylcyclohexane molecules in a given sample exist in chair conformations and about 95. Who are the experts. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. In the given figure various possible chair conformations of 12-dimethylcyclohexane are drawn. Possible chair conformations of 12-dimethylcyclohexane.
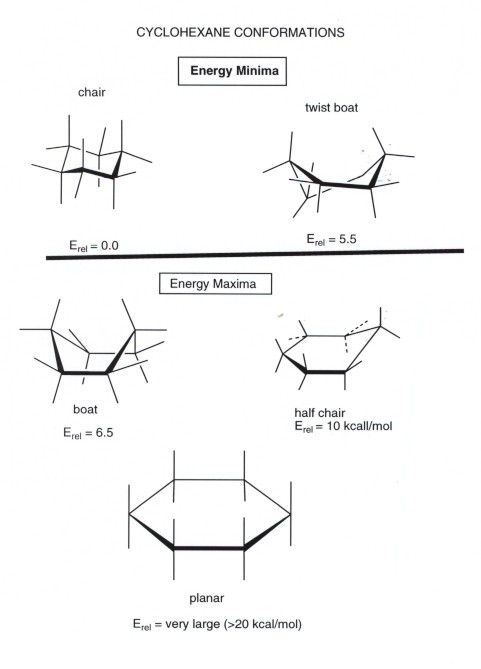 Source: research.cm.utexas.edu
Source: research.cm.utexas.edu
Cyclohexane Conformational Analysis We review their content and use your feedback to keep the. Start with the wedge-dash notation for cis-1-chloro-3-methylcyclohexane which looks like this Since the compound is cis the chloro and the methyl groups must either be on a wedge or on a dash. The ground state conformation of cyclohexane is a fully staggered conformation which is shaped somewhat like a chair. At 25 C 9999 of all molecules in a cyclohexane solution adopt this conformation. The above image shows both of them on wedges. Experts are tested by Chegg as specialists in their subject area.
Please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save or be able to bookmark this blog page in this website. Thank you …










