Chair conformation of l glucose Both sugars 1 and 2 are D-glucose as shown via the chair conformation below. Udeoyamrtoipkelazsram for PEM or udeoyamoilujzednanref for JMF.
Chair Conformation Of L Glucose, Now lets go into more details. O CH 2OH HO HO HO OH D. Beside the correct description of.
 A Equatorial And Axial Directions Of The Ring Shown With E And A Download Scientific Diagram From researchgate.net
A Equatorial And Axial Directions Of The Ring Shown With E And A Download Scientific Diagram From researchgate.net
Another Article :
Although D-glucose has a strong preference for the 4C 1 chair conformation this is not true for all monosaccharides. They found that the chair conformations of the D-glucopyranose units satisfactorily explained the spacings in the X-ray diagram of cellulose whereas the boat con formations did not. Udeoyamrtoipkelazsram for PEM or udeoyamoilujzednanref for JMF. Draw an OH below the ring on C-1 for the α form draw it above the ring for the β form. Both sugars 1 and 2 are D-glucose as shown via the chair conformation below.
Draw the chair conformation of the following.
Add the OH on the anomeric carbon pointing up for the β isomer and pointing down for the ɑ isomer. Previous hypotheses on the conformation of a-l4-polyglucoses were actually based on the assumption that the stability of the two boat Bl and 3B forms of the a-D-glucopyranose ring was comparable to that of the chair Cl form235 In the light of more recent knowledge on conformational analysis28 it appears that the energy difference between a chair and a boat or skew form is far from being small. For the beta-D-glucopyranose it is well known that it takes an all-equatorial chair conformation designated 4 C 1 on figure 1. A B-D - galactopyranose b B-D mannopyranose 7. Chair and the numbers indicate the carbon atoms located above or below the reference plane of the chair made up by C 2 C3 C5 and the ring oxygen.
 Source: socratic.org
Source: socratic.org
How Can I Draw Axial And Equatorial Bonds In Glucose Socratic These structures make it easy to show the configuration at each stereogenic center in the molecule without using wedges and dashes. C-1 is the atom to the right of the oxygen and C-5 is the atom to its left. O CH 2OH HO HO HO OH D. They found that the chair conformations of the D-glucopyranose units satisfactorily explained the spacings in the X-ray diagram of cellulose whereas the boat con formations did not. Glucose is a simple sugar with the molecular formula C6H12O6 which means that it is a molecule that is made of six carbon atoms twelve hydrogen atoms and six oxygen atoms. Draw the Hs and OH groups.
 Source: pediaa.com
Source: pediaa.com
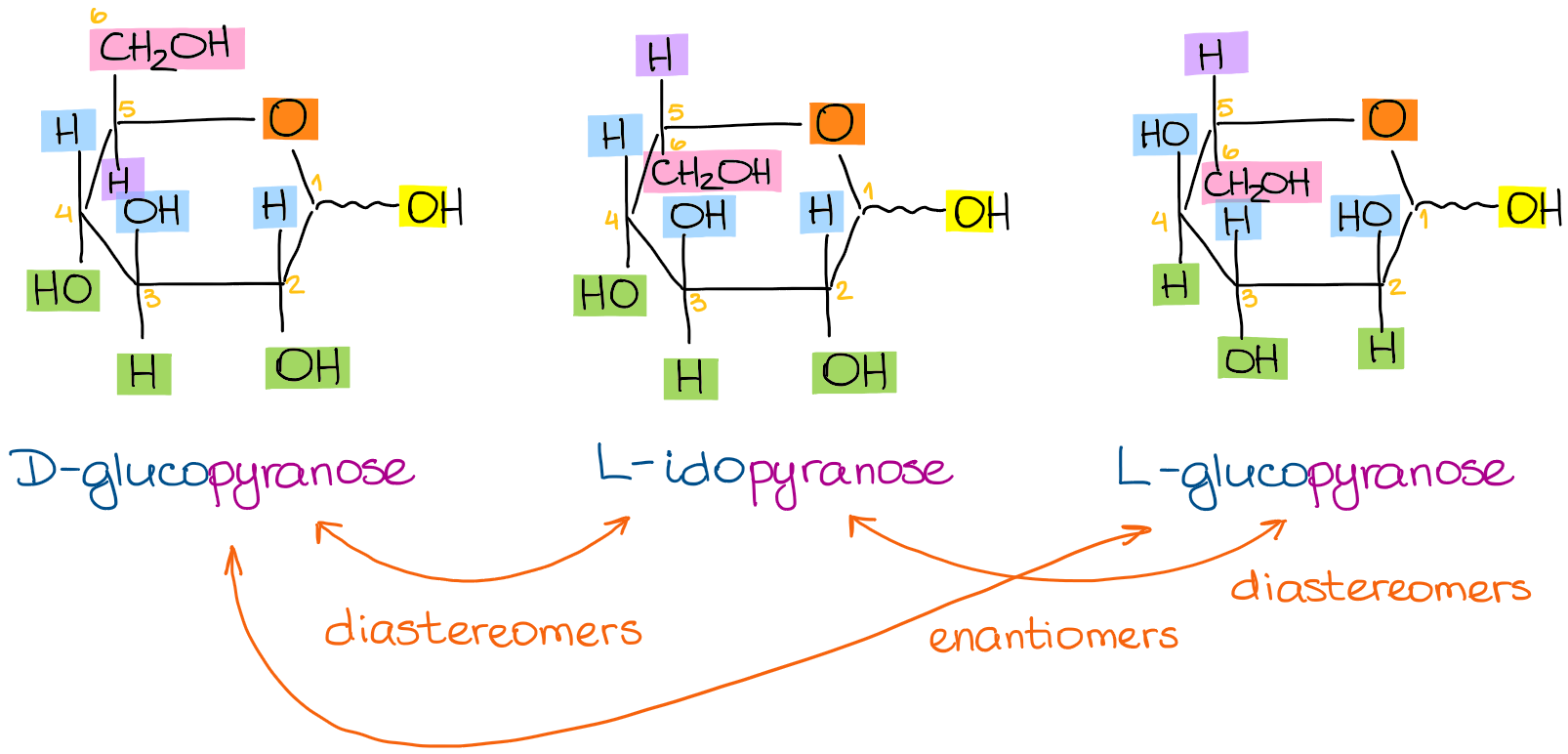
Difference Between D And L Glucose Definition Structure Properties Udeoyamrtoipkelazsram for PEM or udeoyamoilujzednanref for JMF. An alternative chair conformation designated 1 C 4 on figure 1 puts all substituents in axial positions. B acetic anhydride in pyridine b D-galactose b D-allose. In the cyclic forms it exists as a five membered ring called furan of as a six. Beside the correct description of. Glucose can be found in nature as either D-Glucose or L-Glucose.
 Source: researchgate.net
Source: researchgate.net
Sugar Ring Conformations For B Glucopyranosyl Units As Model For The Download Scientific Diagram And by Mohr 13 namely the chair and boat forms could be extended to pyranoid sugars. Chair Conformations of Glucose. Draw the chair conformation of the following. The conformation of the aldopyranosyl ring is also an important issue. O CH 2OH HO HO HO OH D. All the groups on the right side in the Fischer projection point down the groups on the left are pointing up.
 Source: organicchemistrytutor.com
Source: organicchemistrytutor.com
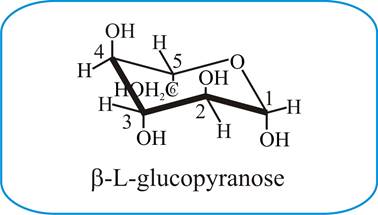
Converting Between Fischer Haworth And Chair Forms Of Carbohydrates Organic Chemistry Tutor For the L-form see L-Glucose. For the L-form see L-Glucose. Draw a basic Haworth projection with the ring oxygen at the top. Although D-glucose has a strong preference for the 4C 1 chair conformation this is not true for all monosaccharides. O CH 2OH HO HO HO OH D. Chair Conformations of Glucose.
 Source: davidmoore.org.uk
Source: davidmoore.org.uk
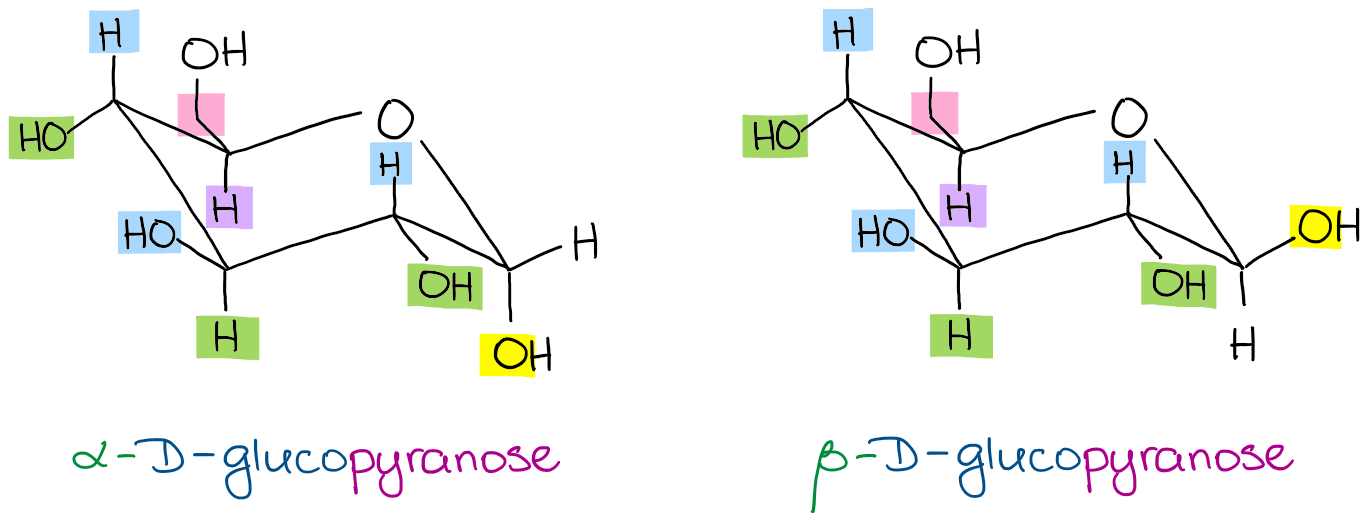
Fungiflex The Untold Story Draw a CH2OH on C-5. Both sugars 1 and 2 are D-glucose as shown below. The acyclic structure of a sugar is commonly drawn as a Fischer projection. Glucose is a simple sugar with the molecular formula C6H12O6 which means that it is a molecule that is made of six carbon atoms twelve hydrogen atoms and six oxygen atoms. The equatorial bonds e are perpendicular to the axis of the ring while axial bonds a are parallel to the axis of the ring. If the hydroxyl group in this anomeric carbon is in the axial position it is said to be alpha glucose.
 Source: organicchemistrytutor.com
Source: organicchemistrytutor.com
Converting Between Fischer Haworth And Chair Forms Of Carbohydrates Organic Chemistry Tutor Chair-boat transitions in single polysaccharide molecules observed with force-ramp AFM Department of Physiology and Biophysics Mayo Foundation Rochester MN 55905 To whom reprint requests may be addressed. Looking at the glucopyranose from above you now can easily draw the chair conformation for it in a general form. 4 pts Convert each of the chair conformations to Fischer projections and name each of the sugars. Draw a basic Haworth projection with the ring oxygen at the top. Each vertical line in the Fischer projection is aligned away from the viewer each horizontal line looks towards the viewer. Previous hypotheses on the conformation of a-l4-polyglucoses were actually based on the assumption that the stability of the two boat Bl and 3B forms of the a-D-glucopyranose ring was comparable to that of the chair Cl form235 In the light of more recent knowledge on conformational analysis28 it appears that the energy difference between a chair and a boat or skew form is far from being small.
 Source: davidmoore.org.uk
Source: davidmoore.org.uk
Fungiflex The Untold Story An alternative chair conformation designated 1 C 4 on figure 1 puts all substituents in axial positions. Chair conformation for each of the sugars present in this carbohydrate. In the chair position the anomeric carbon is the carbon placed right of the oxygen atom bonded in the hexagonal ring. Each vertical line in the Fischer projection is aligned away from the viewer each horizontal line looks towards the viewer. Although D-glucose has a strong preference for the 4C 1 chair conformation this is not true for all monosaccharides. Haworth projection and chair conformation of glucopyranose And just as easily you can show a chair conformations specifically for the α-D-glucopyranose and the β-D-glucopyranose.
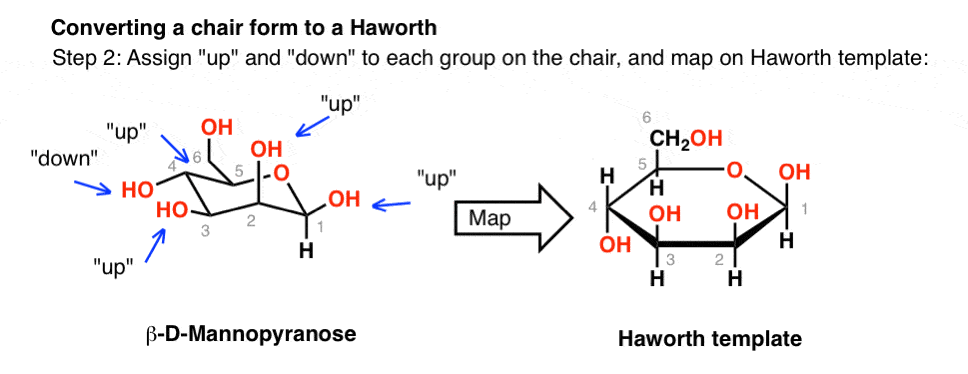 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
The Haworth Projection Master Organic Chemistry Glucose is a sugar and contains hydroxyl groups substituted at five carbons and the 6 th carbon is an aldehydic group. Draw the chair conformation of the following. The most stable conformation of a glucose molecule is called the chair conformation of glucose. Previous hypotheses on the conformation of a-l4-polyglucoses were actually based on the assumption that the stability of the two boat Bl and 3B forms of the a-D-glucopyranose ring was comparable to that of the chair Cl form235 In the light of more recent knowledge on conformational analysis28 it appears that the energy difference between a chair and a boat or skew form is far from being small. In the cyclic forms it exists as a five membered ring called furan of as a six. Each vertical line in the Fischer projection is aligned away from the viewer each horizontal line looks towards the viewer.
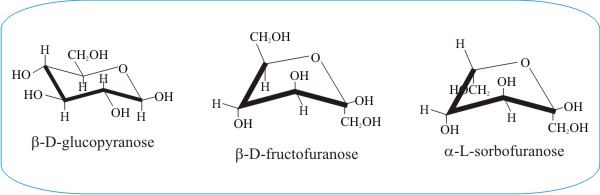 Source: davidmoore.org.uk
Source: davidmoore.org.uk
Fungiflex The Untold Story Glucose is a sugar and contains hydroxyl groups substituted at five carbons and the 6 th carbon is an aldehydic group. For the beta-D-glucopyranose it is well known that it takes an all-equatorial chair conformation designated 4 C 1 on figure 1. The first thing you need to know before drawing the ring-flip of a chair cyclohexane is the correct conformation of the carbon-chain and the orientation of each axial and equatorial group. This article is about the naturally occurring D-form of glucose. All the groups on the right side in the Fischer projection point down the groups on the left are pointing up. C-1 is the atom to the right of the oxygen and C-5 is the atom to its left.
 Source: youtube.com
Source: youtube.com
Chair Conformations Of Glucose Youtube It has equatorial and axial bonds. Draw an OH below the ring on C-1 for the α form draw it above the ring for the β form. Chair Conformations of Glucose - YouTube. Both sugars 1 and 2 are D-glucose as shown below. An alternative chair conformation designated 1 C 4 on figure 1 puts all substituents in axial positions. Draw a CH2OH on C-5.
 Source: sciencedirect.com
Source: sciencedirect.com
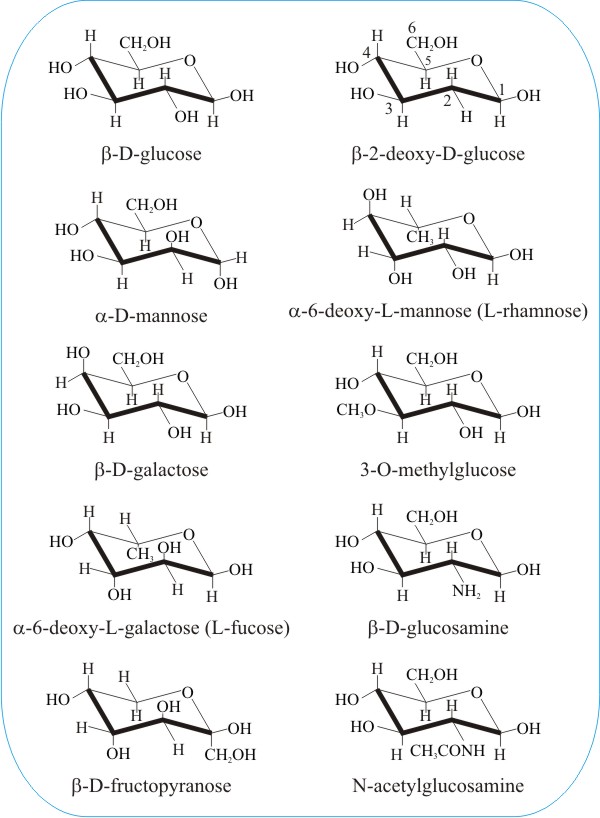
Pyranose An Overview Sciencedirect Topics Glucose circulates in the blood of animals as blood sugar. Convert the Haworth to a chair conformation if needed. And by Mohr 13 namely the chair and boat forms could be extended to pyranoid sugars. Glucose is al aldohexose and is a reducing sugar. Udeoyamrtoipkelazsram for PEM or udeoyamoilujzednanref for JMF. 1 D and L ConfigurationsD and L Configurations.
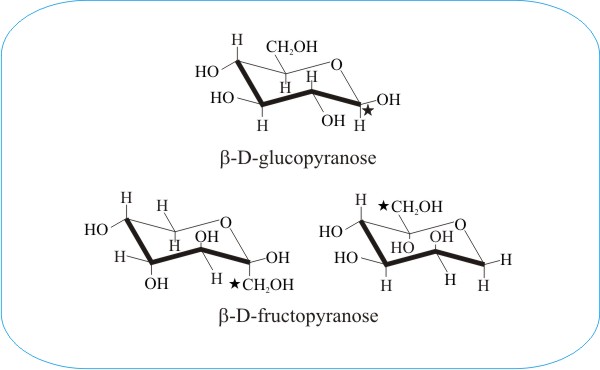 Source: davidmoore.org.uk
Source: davidmoore.org.uk
Fungiflex The Untold Story Udeoyamrtoipkelazsram for PEM or udeoyamoilujzednanref for JMF. The most stable conformation of a glucose molecule is called the chair conformation of glucose. Glucose can be found in nature as either D-Glucose or L-Glucose. What is the full name of the monosaccharide shown below. Draw the chair conformation of the following. These structures make it easy to show the configuration at each stereogenic center in the molecule without using wedges and dashes.
 Source: researchgate.net
Source: researchgate.net
A Equatorial And Axial Directions Of The Ring Shown With E And A Download Scientific Diagram Fischer projection of glucose without stereochemistry shown On the left side you have a glucose structure in the Fischer projection with the stereochemistry shown. The most stable conformation of a glucose molecule is called the chair conformation of glucose. In the chair position the anomeric carbon is the carbon placed right of the oxygen atom bonded in the hexagonal ring. Chair-boat transitions in single polysaccharide molecules observed with force-ramp AFM Department of Physiology and Biophysics Mayo Foundation Rochester MN 55905 To whom reprint requests may be addressed. O CH 2OH HO HO HO OH D. A chair conformation is one of many conformations of a cyclohexane ring and it is most stable.
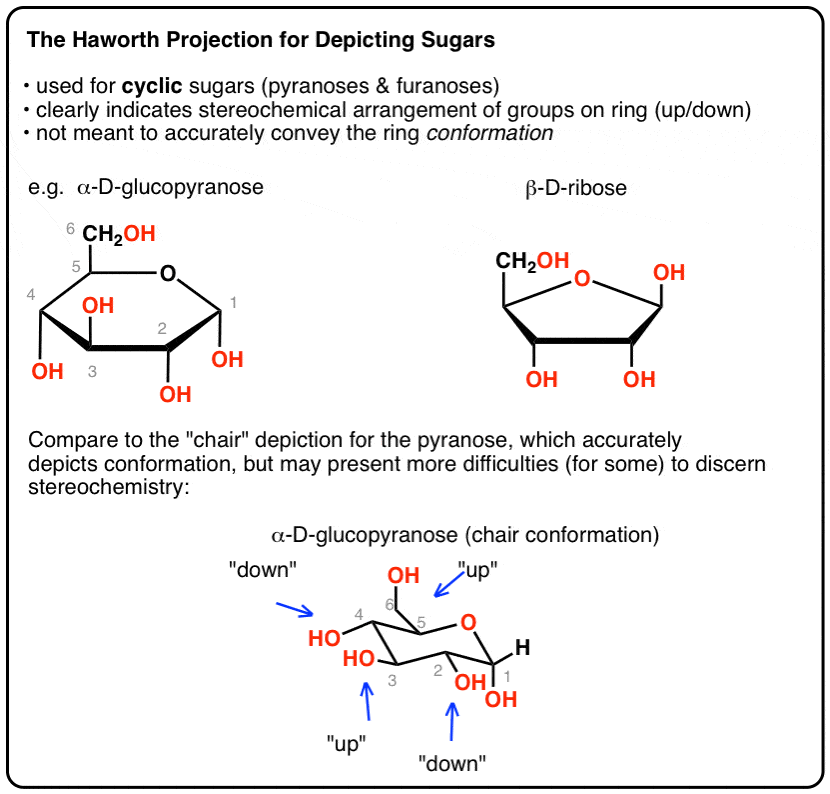 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
The Haworth Projection Master Organic Chemistry Glucose is al aldohexose and is a reducing sugar. Glucose is a simple sugar with the molecular formula C6H12O6 which means that it is a molecule that is made of six carbon atoms twelve hydrogen atoms and six oxygen atoms. Chair Conformations of Glucose - YouTube. Looking at the glucopyranose from above you now can easily draw the chair conformation for it in a general form. A chair conformation is one of many conformations of a cyclohexane ring and it is most stable. For example L-iduronic acid an epimer of glucuronic acid is more stable in the 1C 4 configuration.

Why Does The Most Stable Form Of The Common Sugar Glucose Contain A Six Membered Ring In The Chair Conformation With All The Substituents Equatorial Quora And by Mohr 13 namely the chair and boat forms could be extended to pyranoid sugars. The main difference between D and L Glucose is that D-Glucose rotates plane polarized light clockwise whereas L-Glucose rotates plane polarized light anticlockwise. Both sugars 1 and 2 are D-glucose as shown below. Figure 7 Chair conformations of -D-glucose. Looking at the glucopyranose from above you now can easily draw the chair conformation for it in a general form. Udeoyamrtoipkelazsram for PEM or udeoyamoilujzednanref for JMF.
 Source: bartleby.com
Source: bartleby.com
Chair Conformation Of Glucose Bartleby Edited by Steven Chu. Looking at the glucopyranose from above you now can easily draw the chair conformation for it in a general form. This article is about the naturally occurring D-form of glucose. Glucose can be found in nature as either D-Glucose or L-Glucose. Chair Conformations of Glucose - YouTube. Glucose is a simple sugar with the molecular formula C6H12O6 which means that it is a molecule that is made of six carbon atoms twelve hydrogen atoms and six oxygen atoms.
 Source: semanticscholar.org
Source: semanticscholar.org
Figure 1 From Stereospecificity Of The Glucose Carrier In Sugar Beet Suspension Cells Semantic Scholar This article is about the naturally occurring D-form of glucose. Glucose circulates in the blood of animals as blood sugar. The conformational shape of a pyranose is mainly governed by the relative stability of the two possible chair conformations which are both free of torsional strain but one of. Glucose is a simple sugar with the molecular formula C6H12O6 which means that it is a molecule that is made of six carbon atoms twelve hydrogen atoms and six oxygen atoms. A chair conformation is one of many conformations of a cyclohexane ring and it is most stable. The most stable conformation of a glucose molecule is called the chair conformation of glucose.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
What Are The Differences Between D And L Glucopyranose Chemistry Stack Exchange Looking at the glucopyranose from above you now can easily draw the chair conformation for it in a general form. The most stable conformation of a glucose molecule is called the chair conformation of glucose. The alpha and beta configuration can be given for chair boat conformations of glucose. O CH 2OH HO HO HO OH D. A chair conformation is one of many conformations of a cyclohexane ring and it is most stable. Chair Conformations of Glucose.
Please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark or be able to bookmark this blog page in this website. Thank you …










