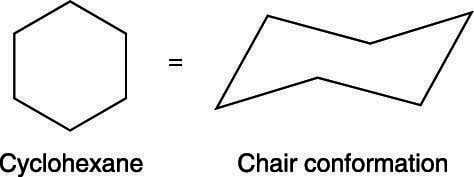
Chair conformation representation At 25 C 9999 of all molecules in a cyclohexane solution adopt this conformation. Some low energy shapes taken by common monosaccharides are shown.
Chair Conformation Representation, C The lower energy chair conformation contains one axial methyl group and one equatorial methyl group. Planar representation to chair conformation. How to draw chairs.
 Chair Conformation And Ring Flips Youtube From youtube.com
Chair Conformation And Ring Flips Youtube From youtube.com
Another Article :
Here we have a model of the cyclohexane molecule and it looks like its a flat hexagon from this perspective but it isnt really if we turn it to the side we can see this is not a planar molecule this is called the chair conformation of cyclohexane and if we stare down these two carbons well be able to see the chair conformation from a Newman projection viewpoint so now you can see that we. Chair conformation lounge chair - used to kick back and relax while you study your organic chemistry Cyclohexane rings are flexible and easily allow partial rotations twists about the C-C single bonds. 19 Convert the flat representation of the cyclohexane molecule at left to the chair conformation templates at right. Cases occur as in β-D-arabinopyranose where both chair conformations are in equilibrium. We should just go over.
A trisubstituted cyclohexane compound is given below in its chair conformation.
If you have not already done so you should. Cyclohexane in the chair conformation has a C3 axis perpendicular to the average plane of the ring three perpendicular C2 axes between the carbons and three v planes each including the C3 axis and one of the C2 axes. And just as easily you can show a chair conformations specifically for the α-D-glucopyranose and the β-D-glucopyranose. Draw the corresponding planar overhead representation using wedges and hashed bonds to indicate the substituent positions. We should just go over.
 Source: pinterest.com
Source: pinterest.com
Image 97167 Forever Alone Science Memes Science Nerd Science Jokes At 25 C 9999 of all molecules in a cyclohexane solution adopt this conformation. Cases occur as in β-D-arabinopyranose where both chair conformations are in equilibrium. C The lower energy chair conformation contains one axial methyl group and one equatorial methyl group. Chair conformations of alpha and beta D-glucopyranose So for as long as you can properly draw the substituents positions in your chair conformation you should be able to easily convert Haworth to chair. At 25 C 9999 of all molecules in a cyclohexane solution adopt this conformation. There is minimal angle strain since each carbon can approximately accommodate the 109o of.
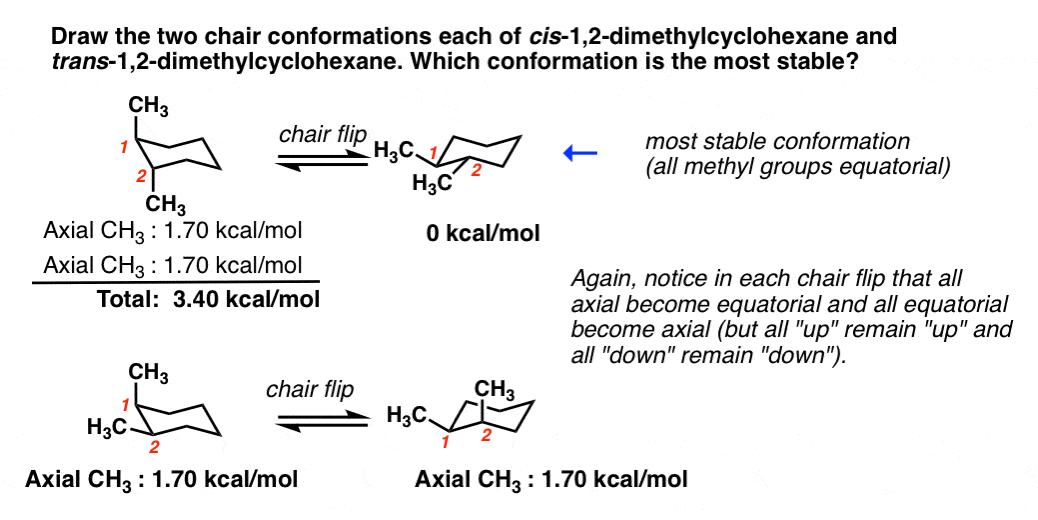 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Cyclohexane Chair Conformation Stability Which One Is Lower Energy Next add a downward-pointing V tip to one end this is the tail of the chair. This conformation of cyclohexane is called the chair conformation be-cause of its resemblance to a lawn chair. The symmetry is D 3d. At 25 C 9999 of all molecules in a cyclohexane solution adopt this conformation. There is minimal angle strain since each carbon can approximately accommodate the 109o of. Figure 2 is a schematic representation of a pyranose ring closure in D-glucose that shows the reorientation at C5 necessary to allow ring formation.
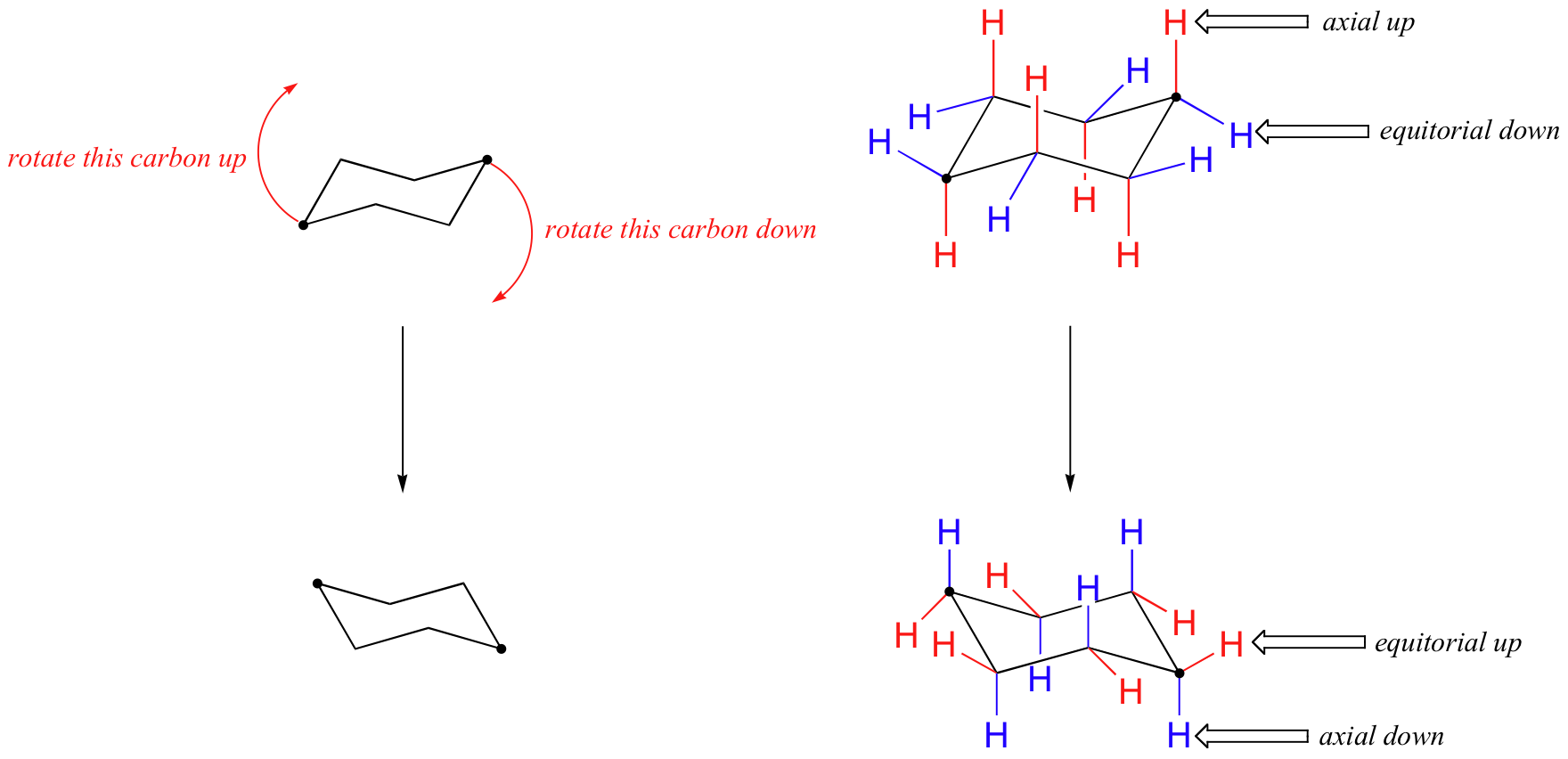 Source: chem.libretexts.org
Source: chem.libretexts.org
4 7 Cyclohexane Conformations Chemistry Libretexts A trisubstituted cyclohexane compound is given below in its chair conformation. Use the guidelines below place substituents in the proper axialequatorial orientation. Conformational representation of the piperidine ring of morphine 1 and analogues meperidine 7 R H R COOC2H and alphaprodine 7 R R 0CC2H. Identify the carbon number for the first substituent if its wedged add it to the up position. Tetrachloroallene has three perpendicular C2. Now that we understand the positions of cyclohexane Im actually going to take like five minutes just to teach you how to draw it.
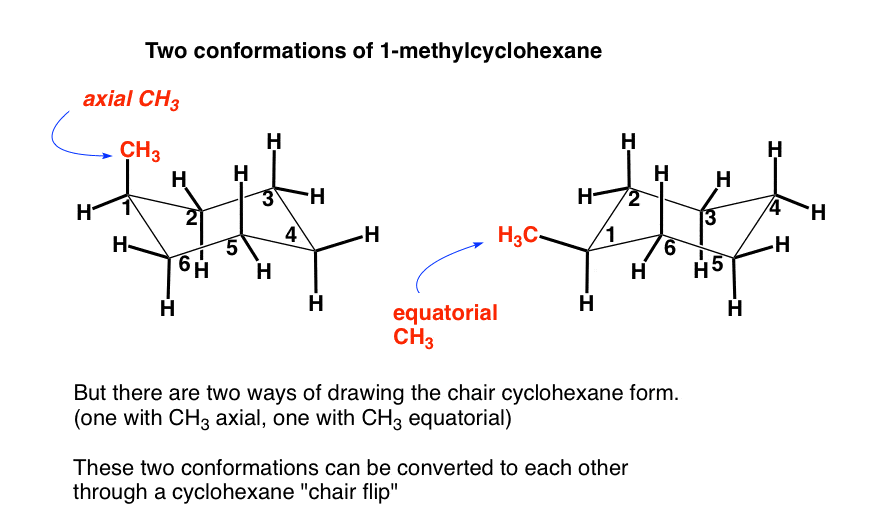 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
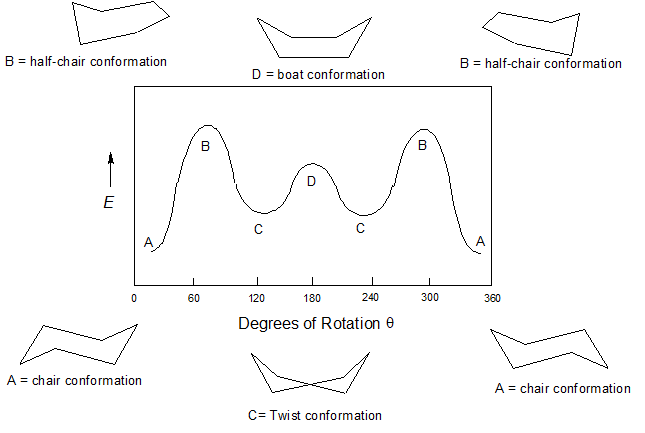
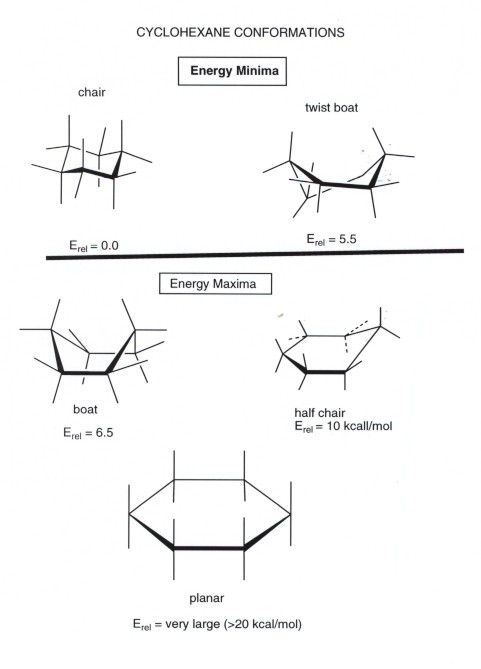
The Cyclohexane Chair Flip Master Organic Chemistry 45 KJmol 63 Boat 32 KJmol Half-chair 45 KJmol Chair Half-chair 45 KJmol Twist-boat 23 KJmol Twist-boat 23 KJmol. D The higher energy chair conformation contains two axial methyl groups. How to draw chairs. The barrier to a chair-chair interconversion is 45 KJmol. 14 Which of the statements below correctly describes the chair conformations of trans-13-. For carbohydrates the position is to the right in a Fischer projection and down in a planar representation.
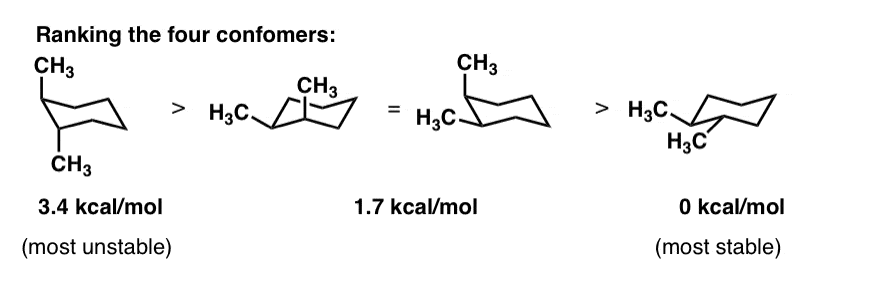 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Cyclohexane Chair Conformation Stability Which One Is Lower Energy Now that we understand the positions of cyclohexane Im actually going to take like five minutes just to teach you how to draw it. Studies of the structure of the cyclic hemiacetal form of D–GLUCOSE using X- ray analysis have shown that actual conformation of the ring are the chair form with the oxygen atom at the upper right represented by conformational formula 3 and 6. There is minimal angle strain since each carbon can approximately accommodate the 109o of. Which of the following is the correct chair representation of the disubstituted cyclohexane in its lowest energy conformation. This conformation of cyclohexane is called the chair conformation be-cause of its resemblance to a lawn chair. 19 Convert the flat representation of the cyclohexane molecule at left to the chair conformation templates at right.
 Source: pinterest.com
Source: pinterest.com
Newman Projections Video Tutorials By Leah4sci Organic Chemistry Organic Chemistry Study Organic Chemistry Reactions Clockwise or counterclockwise doesnt matter as long as you use the same direction for both molecules. In this con-formation of cyclohexane the carbons do not lie in a single plane. Conformational Inversion Ring-Flipping in Cyclohexane Ring flip interchanges the axial and equatorial positions. C The lower energy chair conformation contains one axial methyl group and one equatorial methyl group. There is minimal angle strain since each carbon can approximately accommodate the 109o of. Drawing a Sugars Chair Conformation from a Haworth Structure or Fischer Projection If you have either the Haworth structure or the Fischer projection of the sugar drawing the chair conformation of the sugar is easy as long as you remember the orientations of the axial and equatorial bonds on each carbon atom of the chair conformation.
 Source: in.pinterest.com
Source: in.pinterest.com
Overstock Com Online Shopping Bedding Furniture Electronics Jewelry Clothing More Burodrehstuhl Burostuhl Mobelideen Studies of the structure of the cyclic hemiacetal form of D–GLUCOSE using X- ray analysis have shown that actual conformation of the ring are the chair form with the oxygen atom at the upper right represented by conformational formula 3 and 6. Draw the corresponding planar overhead representation using wedges and hashed bonds to indicate the substituent positions. A trisubstituted cyclohexane compound is given below in its chair conformation. Chair conformations of alpha and beta D-glucopyranose So for as long as you can properly draw the substituents positions in your chair conformation you should be able to easily convert Haworth to chair. For carbohydrates the position is to the right in a Fischer projection and down in a planar representation. Conformational Inversion Ring-Flipping in Cyclohexane Ring flip interchanges the axial and equatorial positions.
 Source: chem.libretexts.org
Source: chem.libretexts.org
4 7 Cyclohexane Conformations Chemistry Libretexts Tetrachloroallene has three perpendicular C2. Which of the following is the correct chair representation of the disubstituted cyclohexane in its lowest energy conformation. Clockwise or counterclockwise doesnt matter as long as you use the same direction for both molecules. C The lower energy chair conformation contains one axial methyl group and one equatorial methyl group. Planar representation to chair conformation. At 25 C 9999 of all molecules in a cyclohexane solution adopt this conformation.
 Source: youtube.com
Source: youtube.com
How To Draw Chair Conformations Of Cyclohexane Rings And Recognize Equivalent Representations Youtube If you have not already done so you should. Scheme in the chair conformation templates drawn in step 3 fill in the substituents on the chair conformations. Substituents represented on wedges are always positioned. In this con-formation of cyclohexane the carbons do not lie in a single plane. E The lower energy chair conformation contains two axial methyl groups. Continued If you know an axial Chlorine Cl adds 2 kJmol of extra energy to cyclohexane an axial Ethyl adds 8 kJmol and an axial Methyl adds 76 kJmol circle the MOST STABLE ie lowest energy conformation below.
 Source: pinterest.com
Source: pinterest.com
Crossed Aldol Condensation Cac As A Feasible Route For Synthesis Of A 1 2 Unsaturated Carbonyl Compound 1 Aldol Condensation Organic Chem Organic Chemistry All carbon centers are equivalent. A trisubstituted cyclohexane compound is given below in its chair conformation. Draw the corresponding planar overhead representation using wedges and hashed bonds to indicate the substituent positions. Which of the following is the correct chair representation of the disubstituted cyclohexane in its lowest energy conformation. Cyclohexane in the chair conformation has a C3 axis perpendicular to the average plane of the ring three perpendicular C2 axes between the carbons and three v planes each including the C3 axis and one of the C2 axes. Six hydrogen centers are poised in axial positions roughly parallel with the C 3 axis and six hydrogen atoms are located near the equator.
 Source: researchgate.net
Source: researchgate.net
Schematic Representation Of Half Chair Conformations 6 Hc 7 And 6 Hc 7 Download Scientific Diagram An oxygen atom is present in the six-membered ring and we are told in the problem that the ring exists in a chair conformation. The chair conformation is the most stable conformer. Schematic representation of the puckering parameters describing the conformations of six-member rings along with five low energy conformations. Chair conformations of alpha and beta D-glucopyranose So for as long as you can properly draw the substituents positions in your chair conformation you should be able to easily convert Haworth to chair. Now that we understand the positions of cyclohexane Im actually going to take like five minutes just to teach you how to draw it. E The lower energy chair conformation contains two axial methyl groups.
 Source: youtube.com
Source: youtube.com
Chair Conformation And Ring Flips Youtube Draw the corresponding planar overhead representation using wedges and hashed bonds to indicate the substituent positions. Here we have a model of the cyclohexane molecule and it looks like its a flat hexagon from this perspective but it isnt really if we turn it to the side we can see this is not a planar molecule this is called the chair conformation of cyclohexane and if we stare down these two carbons well be able to see the chair conformation from a Newman projection viewpoint so now you can see that we. It is only necessary to show bonds to groups that are not hydrogen in your chair conformation. Cyclohexane in the chair conformation has a C3 axis perpendicular to the average plane of the ring three perpendicular C2 axes between the carbons and three v planes each including the C3 axis and one of the C2 axes. Chair conformation lounge chair - used to kick back and relax while you study your organic chemistry Cyclohexane rings are flexible and easily allow partial rotations twists about the C-C single bonds. Identify the carbon number for the first substituent if its wedged add it to the up position.
 Source: researchgate.net
Source: researchgate.net
Schematic Representation Of The Chair Conformation Of The Spirocycles Download Scientific Diagram Start with a blank chair conformation. And just as easily you can show a chair conformations specifically for the α-D-glucopyranose and the β-D-glucopyranose. 19 Convert the flat representation of the cyclohexane molecule at left to the chair conformation templates at right. The chair conformation is the most stable conformer. Scheme in the chair conformation templates drawn in step 3 fill in the substituents on the chair conformations. Tetrachloroallene has three perpendicular C2.
 Source: dummies.com
Source: dummies.com
How To Draw The Chair Conformation Of Cyclohexane Dummies Cases occur as in β-D-arabinopyranose where both chair conformations are in equilibrium. The barrier to a chair-chair interconversion is 45 KJmol. Planar representation to chair conformation. The chair conformation is the most stable conformer. We should just go over. To be graded properly include the hydrogen atoms on the chirality centers asymmetric carbons.
 Source: youtube.com
Source: youtube.com
How To Draw Cyclohexane Chair Conformations And Ring Flips Youtube For carbohydrates the position is to the right in a Fischer projection and down in a planar representation. The most stable conformation of cyclohexane is shown in Fig. Download my free guide 10 S. Conformational Inversion Ring-Flipping in Cyclohexane Ring flip interchanges the axial and equatorial positions. Finally add an upward-pointing V tip to the other end this is the nose of the chair. A trisubstituted cyclohexane compound is given below in its chair conformation.
 Source: pinterest.com
Source: pinterest.com
Pin On Elimination Reactions Conformational Inversion Ring-Flipping in Cyclohexane Ring flip interchanges the axial and equatorial positions. In this con-formation of cyclohexane the carbons do not lie in a single plane. And just as easily you can show a chair conformations specifically for the α-D-glucopyranose and the β-D-glucopyranose. From linear Fischer representation to cyclic structure Haworth and chair conformation In the case of D-glucose the hydroxyl group at C5 reacts intra-molecularly with the. Continued If you know an axial Chlorine Cl adds 2 kJmol of extra energy to cyclohexane an axial Ethyl adds 8 kJmol and an axial Methyl adds 76 kJmol circle the MOST STABLE ie lowest energy conformation below. Conformational Inversion Ring-Flipping in Cyclohexane Ring flip interchanges the axial and equatorial positions.
 Source: research.cm.utexas.edu
Source: research.cm.utexas.edu
Cyclohexane Conformational Analysis Download my free guide 10 S. I know that sounds kind of juvenile but once again there are so many people that struggle to draw this weird shape that its not even worth it not to go over it. Complete the chair conformation by filling in the missing substituent groups. Conformational representation of the piperidine ring of morphine 1 and analogues meperidine 7 R H R COOC2H and alphaprodine 7 R R 0CC2H. The symmetry is D 3d. Cyclohexane in the chair conformation has a C3 axis perpendicular to the average plane of the ring three perpendicular C2 axes between the carbons and three v planes each including the C3 axis and one of the C2 axes.
 Source: youtube.com
Source: youtube.com
Chair Conformations Of Glucose Youtube Planar representation to chair conformation. Conformational Inversion Ring-Flipping in Cyclohexane Ring flip interchanges the axial and equatorial positions. Cases occur as in β-D-arabinopyranose where both chair conformations are in equilibrium. In this con-formation of cyclohexane the carbons do not lie in a single plane. Figure 2 is a schematic representation of a pyranose ring closure in D-glucose that shows the reorientation at C5 necessary to allow ring formation. All carbon centers are equivalent.
Please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save or be able to bookmark this blog page in this website. Thank you …










