Chair conformation resonance Together these features make the chair conformation very stable. At any instant almost all the methylcyclohexane molecules in a given sample exist in chair conformations and about 95 percent.
Chair Conformation Resonance, Surprisingly compound 2 exhibits a different equilibrium between 1C4 chair and 1S3 skew boat conformations and significant flexibility around the pseudoglycosidic linkage when in solution. All the carbon-hydrogen bonds are also fully staggered eliminating the torsional strain. For this reason we favor a half-chair conformation for the dihydropyran ring or a preponderance of the half-chair should it exist in.
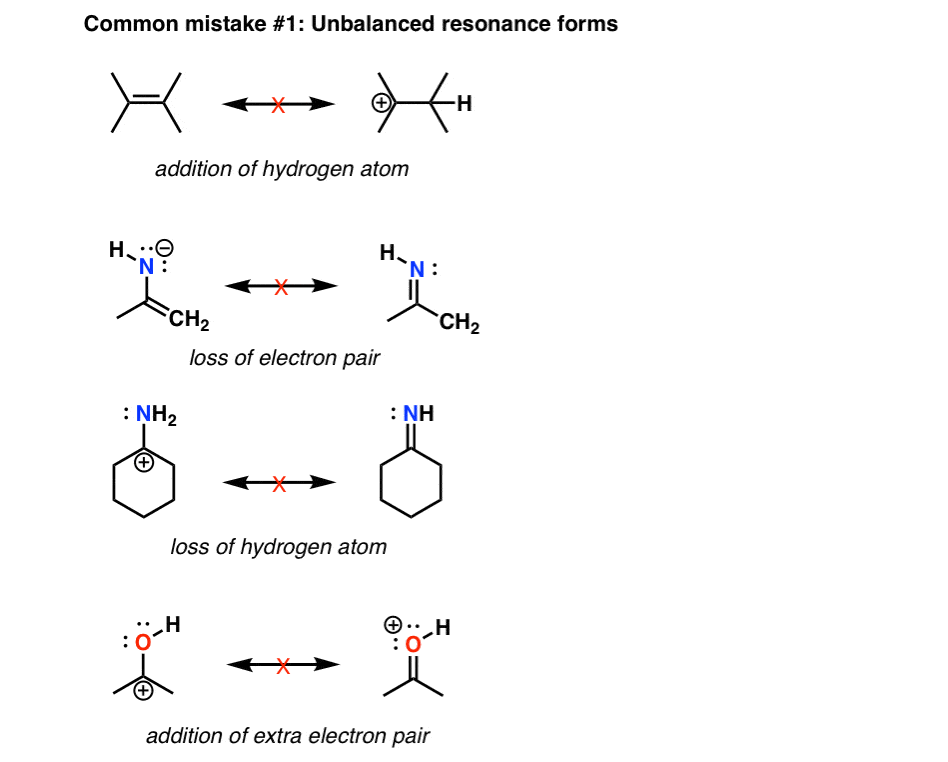 Drawing Resonance Structures 3 Common Mistakes To Avoid From masterorganicchemistry.com
Drawing Resonance Structures 3 Common Mistakes To Avoid From masterorganicchemistry.com
Another Article :
With this conformation the bond angles are 1109 degrees much closer to the ideal 1095 degrees. Dont forget up stays up and down stays down. The most stable conformation of the cyclohexane ring is called the chair conformation. The chair conformation is a six-membered ring in which atoms 2 3 5 and 6 lie in the same plane atom 1 lies above the plane and atom 4 lies below the plane. Upon ring flipping the axial and equatorial bonds interchange their positions.
The most stable conformation of cyclohexane is called chair conformation since it somewhat resembles a chair.
Rank the resonance structures below from most to least important. For this reason we favor a half-chair conformation for the dihydropyran ring or a preponderance of the half-chair should it exist in. All the carbon-hydrogen bonds are also fully staggered eliminating the torsional strain. Dont forget up stays up and down stays down. Usually chair conformation is the most stable conformation and at room temperature about 9999 of cyclohexane in a mixture of different conformation exists in this conformation.
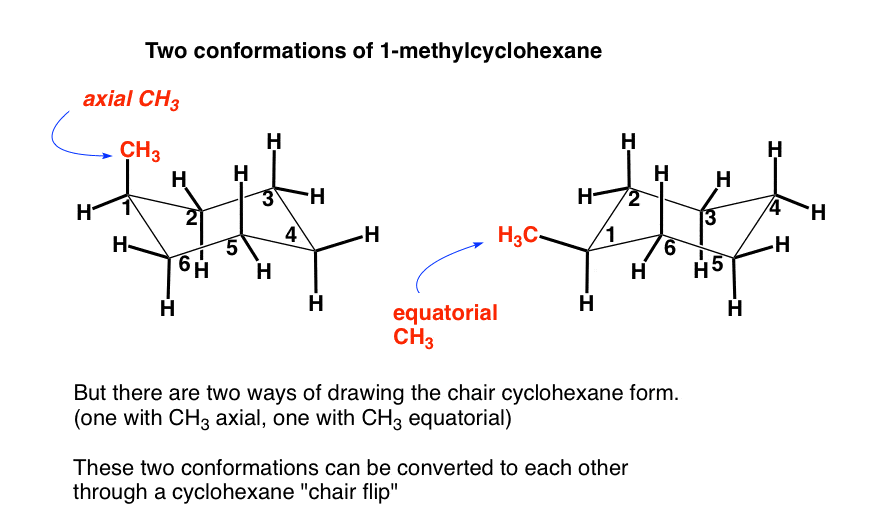 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
The Cyclohexane Chair Flip Master Organic Chemistry Chair form gives the least strain - or in other words - the most energetically favourable conformation. The arrangement of the molecules is such that they are butting heads the least. There are two different chair conformations that rapidly interchange via a pathway that passes through many different conformations including a high-energy half-chair conformation as well as a twist boat and boat conformations. The chair conformation has no torsional strain all the hs are staggered none are eclipsed. CH3 Question 5 Label 4 different functional groups found in the structure. The chair conformation has alternating axial up axial down so once you have that single axial substituent move on to.
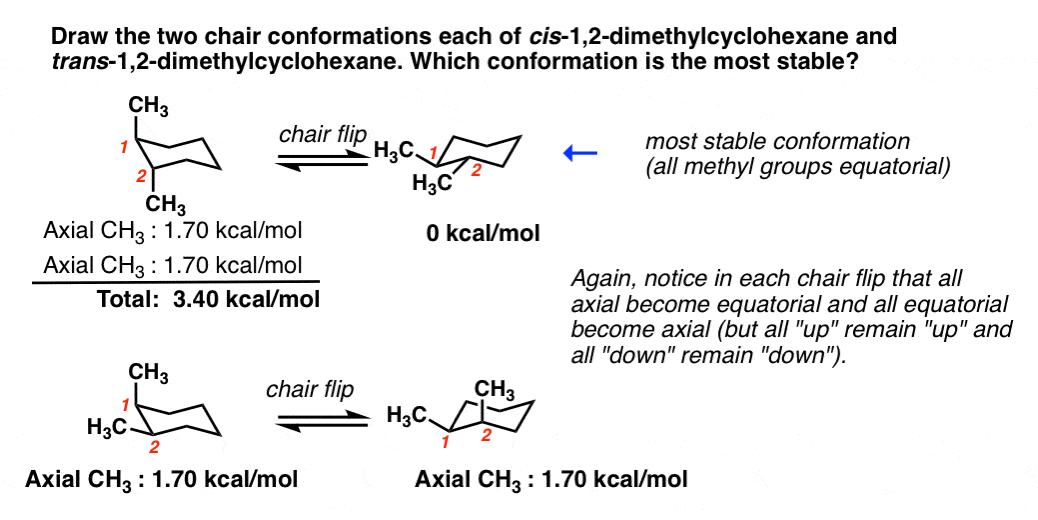 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Cyclohexane Chair Conformation Stability Which One Is Lower Energy The chair conformation has alternating axial up axial down so once you have that single axial substituent move on to. Resonance T T T Cl could eq ually well be drawn in the hashed spot A. If the tert-butyl group is placed in the axial bond then the chair has the highest energy or the least stable conformation. There are two different chair conformations that rapidly interchange via a pathway that passes through many different conformations including a high-energy half-chair conformation as well as a twist boat and boat conformations. The tert-butyl group needs to be placed in the equatorial bond as it is the lowest energy or highest stability conformation. CH3 Question 5 Label 4 different functional groups found in the structure.
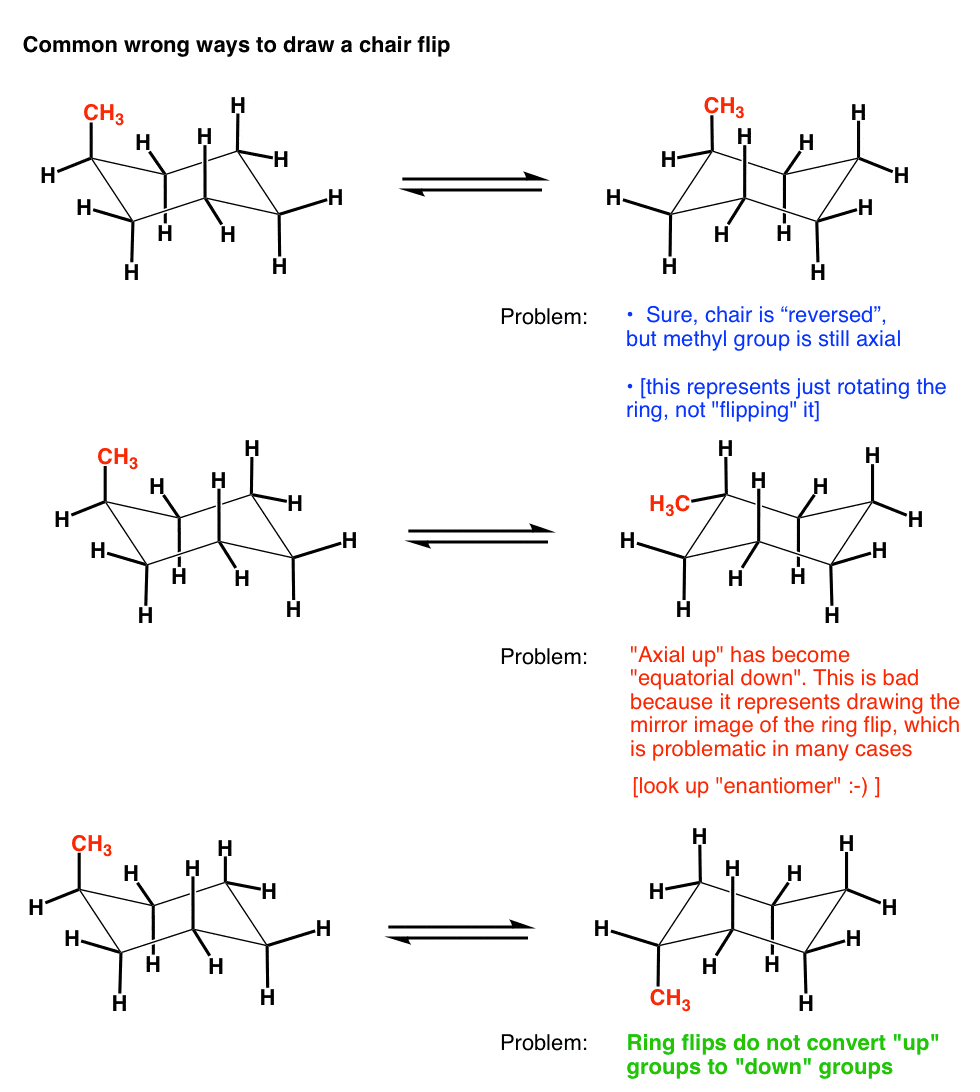 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
The Cyclohexane Chair Flip Master Organic Chemistry Which chair conformation is the most stable conformation of all cis-124-trimethylcyclohexane. Moreover the symmetry of chair conformation is D 3d while boat symmetry has the symmetry C 2v. 1 Draw the product of the reaction in chair form 3 12. If the tert-butyl group is placed in the axial bond then the chair has the highest energy or the least stable conformation. It is concluded that ψ-pelletierine exists essentially in a chairchair conformation with distortions in the 4-piperidone and probably in the piperidine ring. With this conformation the bond angles are 1109 degrees much closer to the ideal 1095 degrees.
 Source: teachthemechanism.com
Source: teachthemechanism.com
Rules Of Thumb Rots For Chair Conformations And Substituent Stability Teach The Mechanism In the chair conformation of cyclohexane all the carbons are at 1095º bond angles so no angle strain applies. Draw the following in the lowest energy chair conformation. Draw the lowest energy chair conformation for each of the following cyclohexane structures. Together these features make the chair conformation very stable. The chair conformation is a six-membered ring in which atoms 2 3 5 and 6 lie in the same plane atom 1 lies above the plane and atom 4 lies below the plane. The structure is highly stabilized due to the resonance structure.
 Source: youtube.com
Source: youtube.com
How To Draw Cyclohexane Chair Conformations And Ring Flips Youtube Chair form gives the least strain - or in other words - the most energetically favourable conformation. Lone pairs are not explicitly shown think about the number of lone pairs implied by the bond line structures. Draw BOTH chairs and compare energy and stability. Upon ring flipping the axial and equatorial bonds interchange their positions. Every carbon on the chair conformation has 1 substituent. 2 Draw a planar cyclohexane structure of the chair product keeping the H atom at the north-most position 4.
 Source: researchgate.net
Source: researchgate.net
0 88 Hz And 0 01 Hz In The 4 Twisted Chair Conformation In Order Download Scientific Diagram The X-ray crystal structure of its difluoromethylene derivative 2 similarly shows a 4C1 chair conformation. Which chair conformation is the most stable conformation of all cis-124-trimethylcyclohexane. Surprisingly compound 2 exhibits a different equilibrium between 1C4 chair and 1S3 skew boat conformations and significant flexibility around the pseudoglycosidic linkage when in solution. 1 Draw the product of the reaction in chair form 3 12. Draw the ring flip for each of the following chair conformations. The arrangement of the molecules is such that they are butting heads the least.
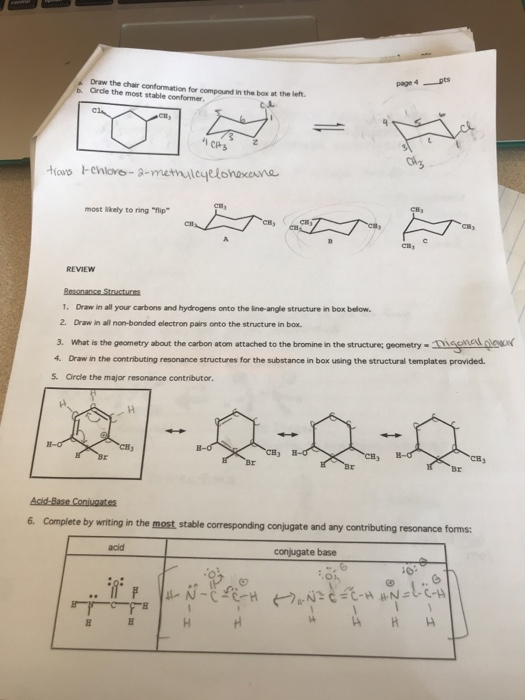
Solved Draw The Chair Conformation For Compound In The Box Chegg Com There will always be some strain no matter what conformation the molecule is in. Surprisingly compound 2 exhibits a different equilibrium between 1C4 chair and 1S3 skew boat conformations and significant flexibility around the pseudoglycosidic linkage when in solution. There are two different chair conformations that rapidly interchange via a pathway that passes through many different conformations including a high-energy half-chair conformation as well as a twist boat and boat conformations. Besides boat conformation tends. The tert-butyl group needs to be placed in the equatorial bond as it is the lowest energy or highest stability conformation. Usually chair conformation is the most stable conformation and at room temperature about 9999 of cyclohexane in a mixture of different conformation exists in this conformation.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Drawing Resonance Structures 3 Common Mistakes To Avoid However keep in mind that the geometry of the cyclohexane needs to be. There will always be some strain no matter what conformation the molecule is in. The chair conformation is customarily drawn either as 2 or as 3 which are mirror images of each other. Lone pairs are not explicitly shown think about the number of lone pairs implied by the bond line structures. Moreover the symmetry of chair conformation is D 3d while boat symmetry has the symmetry C 2v. Alternate your axial substituents up and down all the way around your cyclohexane.
 Source: chegg.com
Source: chegg.com
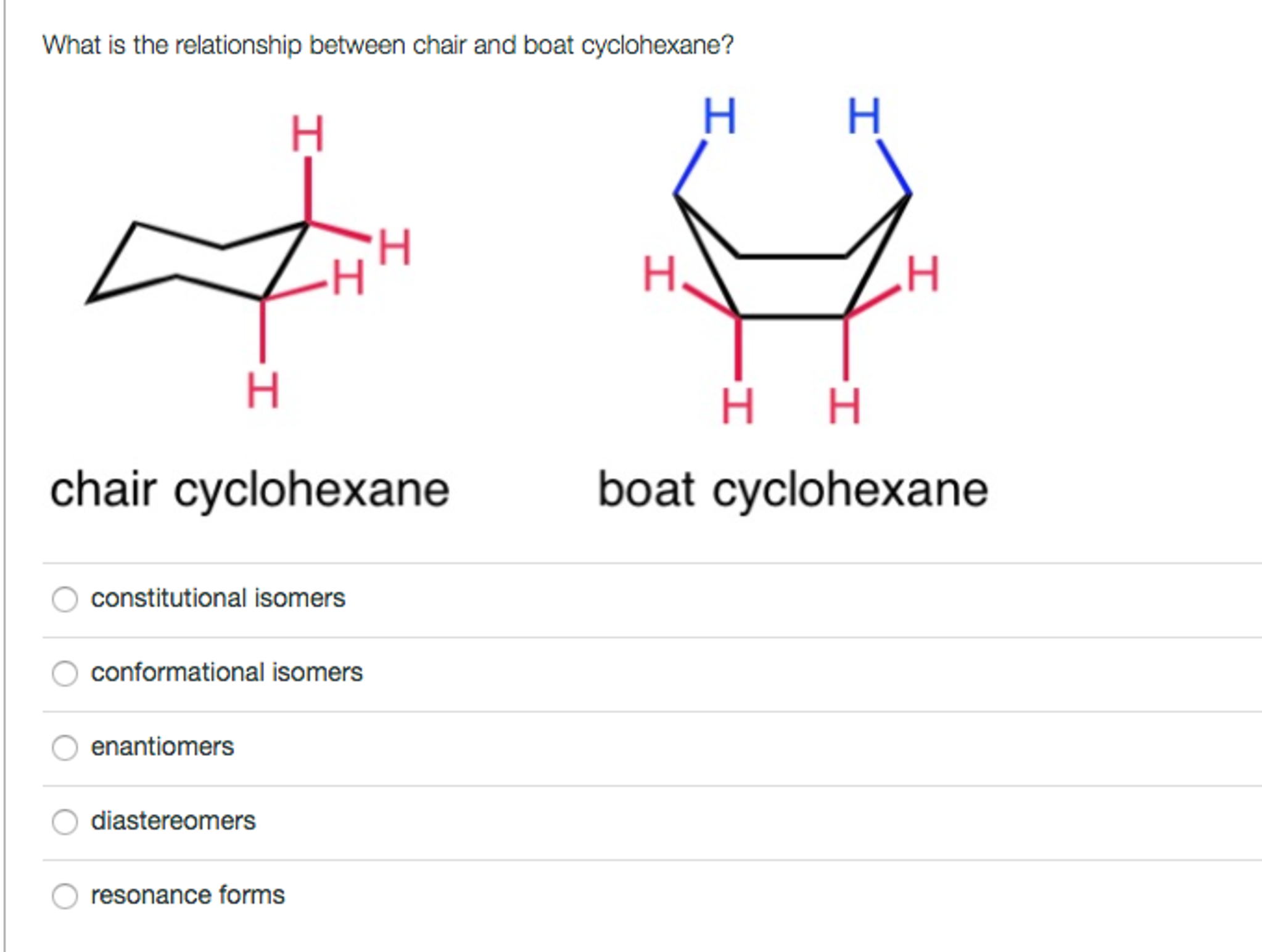
Solved Relationship Between Chair And Boat Chegg Com Besides boat conformation tends. Lone pairs are not explicitly shown think about the number of lone pairs implied by the bond line structures. This chair-chair interconversion that leads to the generation of two equivalent energy forms is known as ring flipping. Usually chair conformation is the most stable conformation and at room temperature about 9999 of cyclohexane in a mixture of different conformation exists in this conformation. Draw the lowest energy chair conformation for each of the following cyclohexane structures. The chair conformation has no torsional strain all the hs are staggered none are eclipsed.
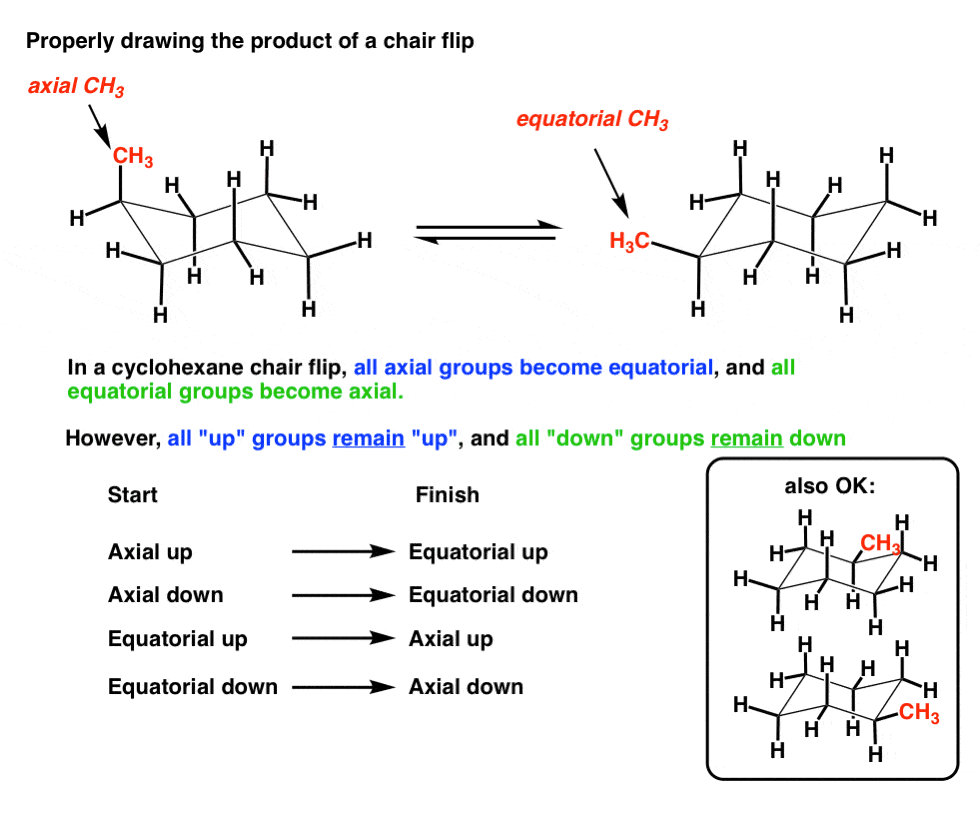 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
The Cyclohexane Chair Flip Master Organic Chemistry Forcing it to go chair or boat conformation will require a very high energy since youre destroying the stabilized structure of benzene. 2 Draw a planar cyclohexane structure of the chair product keeping the H atom at the north-most position 4. Every carbon on the chair conformation has 1 substituent. Does Benzene Have Chair And Boat Conformations. Which chair conformation is the most stable conformation of all cis-124-trimethylcyclohexane. Draw the lowest energy chair conformation for each of the following cyclohexane structures.
 Source: sciencedirect.com
Source: sciencedirect.com
Ring Flipping An Overview Sciencedirect Topics This suggests that the conformation about the C3-C4 bond of the dihydropyran ring is similar to the staggered conformation in chair cyclohexanes. Benzene is predominantly planar. Usually chair conformation is the most stable conformation and at room temperature about 9999 of cyclohexane in a mixture of different conformation exists in this conformation. Alternate your axial substituents up and down all the way around your cyclohexane. Hence the angle strain in the chair conformation is very small. In the chair conformation of cyclohexane all the carbons are at 1095º bond angles so no angle strain applies.
 Source: researchgate.net
Source: researchgate.net
Cena In Its Two Stable Conformations A The 2 H 3 Half Chair Download Scientific Diagram The tert-butyl group needs to be placed in the equatorial bond as it is the lowest energy or highest stability conformation. The tert-butyl group needs to be placed in the equatorial bond as it is the lowest energy or highest stability conformation. This chair-chair interconversion that leads to the generation of two equivalent energy forms is known as ring flipping. NHz HzN Question 4 Convert the following Newman projection to line-angle. This suggests that the conformation about the C3-C4 bond of the dihydropyran ring is similar to the staggered conformation in chair cyclohexanes. RESONANCE September 2014 849 Method for converting chair-like transition states into zig-zag projections Scheme 3 Consider the aldol reaction of Z-enolates Scheme 1.
 Source: researchgate.net
Source: researchgate.net
4 C 1 Chair Conformation Of A Hexopyranose And Newman Projections Of Download Scientific Diagram Draw the lowest energy chair conformation for each of the following cyclohexane structures. The X-ray crystal structure of its difluoromethylene derivative 2 similarly shows a 4C1 chair conformation. Every carbon on the chair conformation has 1 substituent. While 3-α-granatanol exhibits a chairboat equilibrium with the boat form predominating in the 4-piperdinol ring 3-β-granatanol seems to occur mainly in a chairchair conformation. Alternate your axial substituents up and down all the way around your cyclohexane. The arrangement of the molecules is such that they are butting heads the least.
 Source: researchgate.net
Source: researchgate.net
Chair Conformations A C And Newman Projections B D Of Download Scientific Diagram Benzene is predominantly planar. The chair conformation is a six-membered ring in which atoms 2 3 5 and 6 lie in the same plane atom 1 lies above the plane and atom 4 lies below the plane. The X-ray crystal structure of its difluoromethylene derivative 2 similarly shows a 4C1 chair conformation. In methylcyclohexane the chair conformation in which the large methyl group is equatorial is the most stable and therefore the most populated of all possible conformations. The chair conformation has no torsional strain all the hs are staggered none are eclipsed. Does Benzene Have Chair And Boat Conformations.
 Source: researchgate.net
Source: researchgate.net
Chair Conformations A C And Newman Projections B D Of Download Scientific Diagram Alternate your axial substituents up and down all the way around your cyclohexane. Use wedge-dash as needed. The chair conformation is customarily drawn either as 2 or as 3 which are mirror images of each other. 1 Draw the product of the reaction in chair form 3 12. The X-ray crystal structure of its difluoromethylene derivative 2 similarly shows a 4C1 chair conformation. In the chair comformation the internal bond angle at a carbon atom is 1114º very close to the ideal value 1095º.
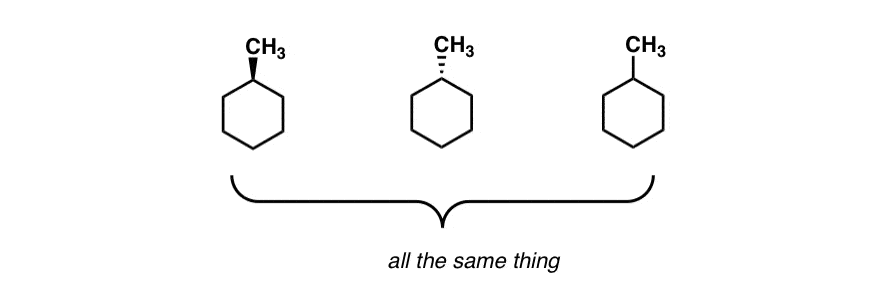 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
The Cyclohexane Chair Flip Master Organic Chemistry 1 Draw the product of the reaction in chair form 3 12. With this conformation the bond angles are 1109 degrees much closer to the ideal 1095 degrees. Rank the resonance structures below from most to least important. Resonance T T T Cl could eq ually well be drawn in the hashed spot A. A chair conformation of cyclohexane can undergo a conformational change into another chair conformer by the partial rotation of C-C bonds. Due to this reason chair conformation is stable than boat conformation.
 Source: youtube.com
Source: youtube.com
Evaluating Relative Stability Of Chair Conformers Youtube By taking on chair conformation there is zero angle strain and zero torsional no eclipsing C. If it has ideal angles then some eclipsing and torsional strain destabilizes it. Upon ring flipping the axial and equatorial bonds interchange their positions. In methylcyclohexane the chair conformation in which the large methyl group is equatorial is the most stable and therefore the most populated of all possible conformations. The chair conformation has alternating axial up axial down so once you have that single axial substituent move on to. Rank the resonance structures below from most to least important.
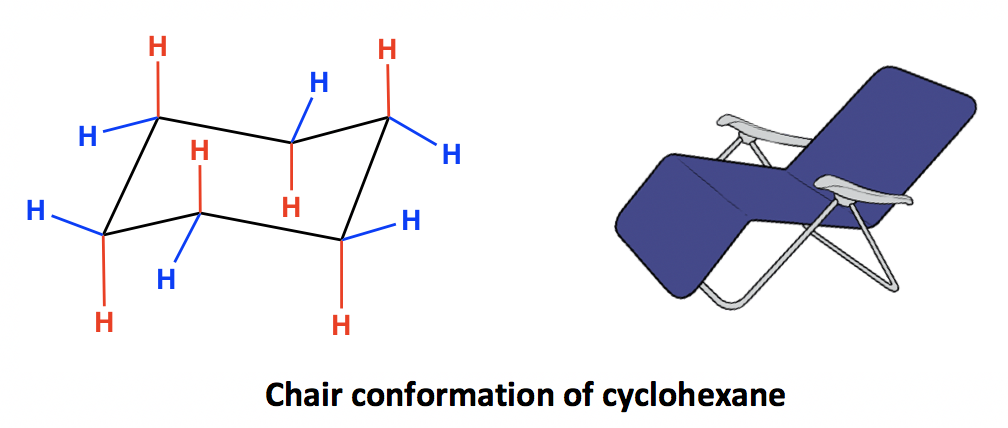 Source: kpu.pressbooks.pub
Source: kpu.pressbooks.pub
4 3 Conformation Analysis Of Cyclohexane Organic Chemistry Chair form gives the least strain - or in other words - the most energetically favourable conformation. Chair form gives the least strain - or in other words - the most energetically favourable conformation. Hence the angle strain in the chair conformation is very small. 1 Draw the product of the reaction in chair form 3 12. The most stable conformation of the cyclohexane ring is called the chair conformation. Resonance T T T Cl could eq ually well be drawn in the hashed spot A.
Please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark or be able to bookmark this blog page in this website. Thank you …










