Chair conformation with double bond Start with a chair cyclohexane. Because 2-3 and 5-6 are side by side while 1-2 and 4-5 are not side by side even though both pairs are parallel.
Chair Conformation With Double Bond, Most of the time the structure exists in what is called the chair conformation. A Selection of AG Values for the Change from Axial to Equatorial Orientation of Substituents for Monosubstituted Cyclohexanes. This conformation is called the chair because it looks sort of like a reclining lounge chair as shown here.

Another Article :
Complete a 2nd chair. There are only boat-like conformations with relative energies within a 06 kcalmol window and a maximum barrier for pseudorotation of Δ G 427. The strain energies of cyclenes with one double bond in the ring ranging from C 6 to C 9 have been calculated as a function of various geometric parameters. Chair Conformation and Ring Flips - YouTube. Bicyclic compounds that have bridgehead double bonds within larger rings are more stable and can be isolated.
Additionally either bond-line or Newman formulas reveal that the two hydrogen atoms at each CH2 are not the same.
Like the corresponding trans-cycloalkenes bicyclic compounds containing bridgehead double bonds solely within small rings are too unstable to isolate. The chair structure consists of a six-membered ring where every C-C bond exists in a staggered conformation. Cyclohexane and the Chair Structure. Additionally either bond-line or Newman formulas reveal that the two hydrogen atoms at each CH2 are not the same. Take a molecule i made up rn 1-hydroxy-3-methyl-5-cyclohexene this drawn is in the shape of a ring has a hydroxy at the top and a methane at the 3rd spot and a double bond on the fifth and 6th carbon could i draw this on a chair conformation i assume not because of the pi bond but i am not sure.
 Source: reddit.com
Source: reddit.com
Can A Chair Conformation Include A Double Bond On A Ring Chemhelp The 6 rm6 6 membered rings of decalin similar form of cyclohexane which are estimated to be more stable in the chair form. The Chair Conformation The stability data in Table 71 require that the bond angles in cyclohexane must be essentially the same as the bond angles in an alkanevery close to the ideal 1095 tetrahedral angle. Start with a chair cyclohexane. For each chair conformer add the energy of all the groups on axial position. With the double bond in α-position of the keto function the resulting delocalisation and hence flattening of the adjacent atoms in the ring prohibit the occurrence of a chair conformation. Add two vicinal bonds.
 Source: pinterest.com
Source: pinterest.com
Antiperiplanar Relationships The E2 Reaction And Cyclohexane Rings Organic Chemistry Organic Chemistry Study Chemistry Lessons The perfect ring Conformations of Cyclohexane Chair conformation H H H H H H H H H H H H Conformations of Cyclohexan Chair conformation Equatorial hydrogens H H H H H. Can a chair conformation include a double bond on a ring. The Chair Conformation The stability data in Table 71 require that the bond angles in cyclohexane must be essentially the same as the bond angles in an alkanevery close to the ideal 1095 tetrahedral angle. Three hydrogen atoms point straight up and three Hs point straight down at. The most stable conformation of cyclohexane is the chair form shown to the right. The strain energies of cyclenes with one double bond in the ring ranging from C 6 to C 9 have been calculated as a function of various geometric parameters.
 Source: researchgate.net
Source: researchgate.net
Cena In Its Two Stable Conformations A The 2h3 Half Chair Download Scientific Diagram One is the cis-decalin in which the hydrogens present at the ring junction are on the same side and the other is the trans-decalin in which the ring-junction hydrogens are on the. The Newman diagram is saying that the head of the chair is 3 and the legs of the chair is 6. The 6 rm6 6 membered rings of decalin similar form of cyclohexane which are estimated to be more stable in the chair form. The perfect ring Conformations of Cyclohexane Chair conformation H H H H H H H H H H H H Conformations of Cyclohexan Chair conformation Equatorial hydrogens H H H H H. The outer bonds called equatorial bonds flip into vertical bonds. With the double bond in α-position of the keto function the resulting delocalisation and hence flattening of the adjacent atoms in the ring prohibit the occurrence of a chair conformation.
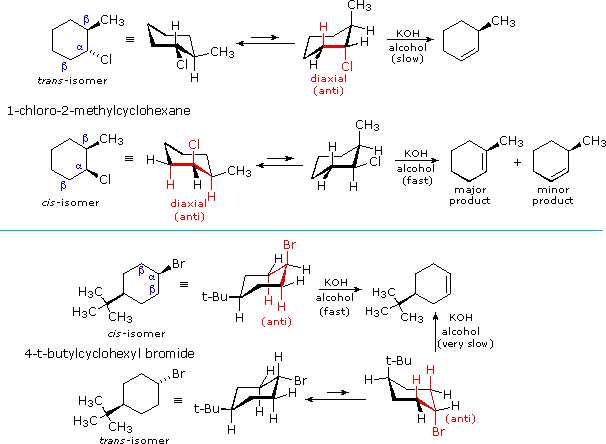 Source: chem.libretexts.org
Source: chem.libretexts.org
7 16 E2 Regiochemistry And Cyclohexane Conformations Chemistry Libretexts Three hydrogen atoms point straight up and three Hs point straight down at. Ii Substituents on chair conformers prefer to occupy equatorial positions due to the increased steric hindrance of axial locations. Three hydrogen atoms point straight up and three Hs point straight down at. Additionally either bond-line or Newman formulas reveal that the two hydrogen atoms at each CH2 are not the same. Add two vicinal bonds. Complete a 2nd chair.
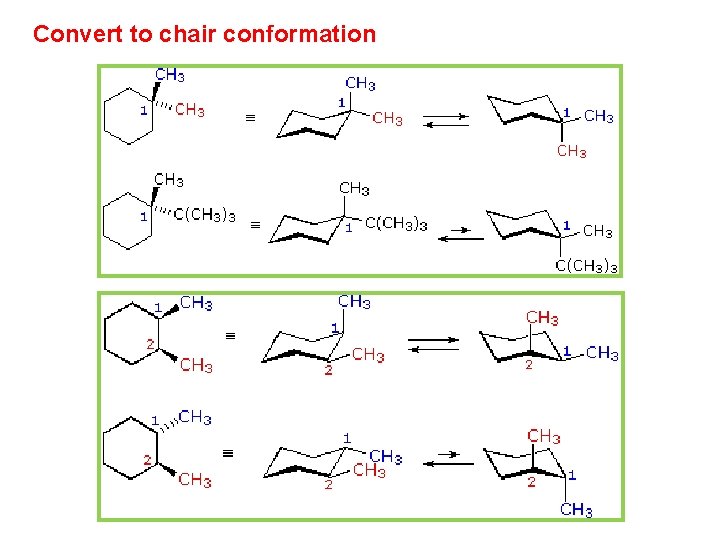 Source: slidetodoc.com
Source: slidetodoc.com
Chapter 4 Alkanes Alkenes And Alkynes Nomenclature Conformational And now the stabilities. This conformation is called the chair because it looks sort of like a reclining lounge chair as shown here. Start with a chair cyclohexane. Most of the time the structure exists in what is called the chair conformation. If the bond angles were significantly distorted from tetrahedral we would expect to see a greater heat of formation. Although the hydrocarbon cyclohexane is typically drawn as if it were flat in reality the structure is not flat at all.
 Source: researchgate.net
Source: researchgate.net
Conformational Analysis Involving A Double Bond The C-C-C bonds are very close to 1095 o so it is almost free of angle strain. One is the cis-decalin in which the hydrogens present at the ring junction are on the same side and the other is the trans-decalin in which the ring-junction hydrogens are on the. Cyclohexane and the Chair Structure. The Newman diagram is saying that the head of the chair is 3 and the legs of the chair is 6. The 6 rm6 6 membered rings of decalin similar form of cyclohexane which are estimated to be more stable in the chair form. The chair structure consists of a six-membered ring where every C-C bond exists in a staggered conformation.
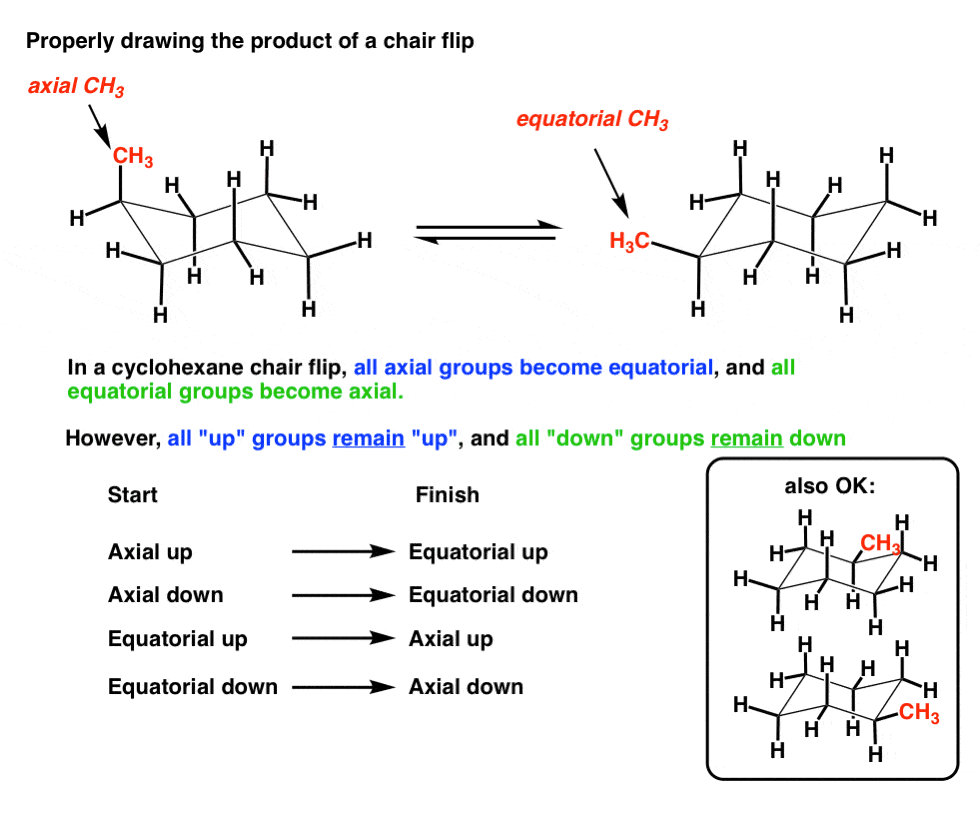 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
The Cyclohexane Chair Flip Master Organic Chemistry The chair structure of cyclohexane is considered to be the perfect conformation. Three hydrogen atoms point straight up and three Hs point straight down at. If the bond angles were significantly distorted from tetrahedral we would expect to see a greater heat of formation. Number the ring and draw any chair conformation of the compound. Half-chair twist boat The chair form of cyclohexane is flexible and may be flipped into other chair forms. There are only boat-like conformations with relative energies within a 06 kcalmol window and a maximum barrier for pseudorotation of Δ G 427.
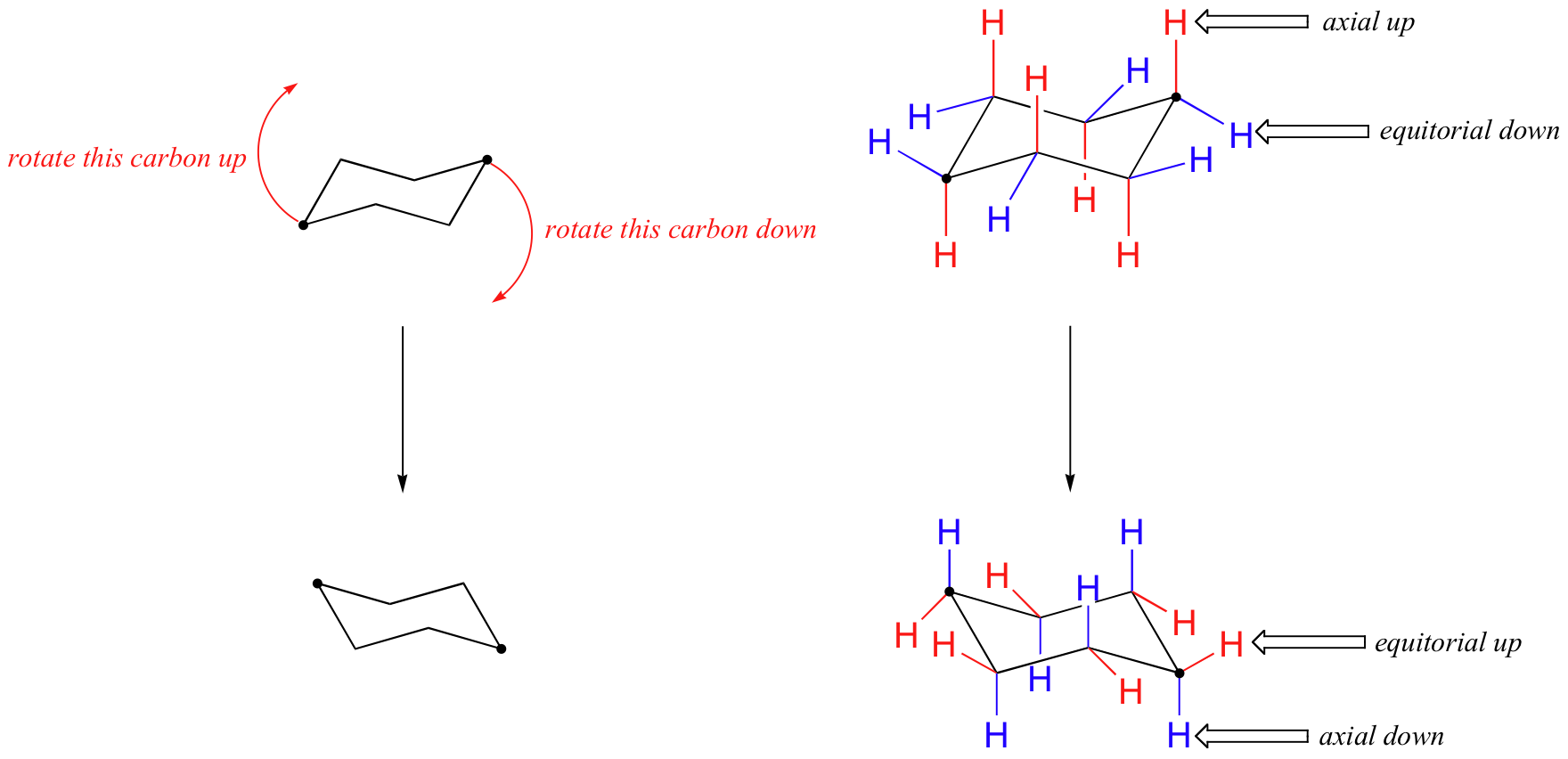 Source: chem.libretexts.org
Source: chem.libretexts.org
4 7 Cyclohexane Conformations Chemistry Libretexts Cyclohexane adopts a CHAIR CONFORMATION that has no ring strain and no angle strain. Cyclohexane adopts a CHAIR CONFORMATION that has no ring strain and no angle strain. Half-chair twist boat The chair form of cyclohexane is flexible and may be flipped into other chair forms. It is also a fully staggered conformation and so is free of torsional strain. A fair agreement is found. The most stable conformation of cyclohexane is the chair form shown to the right.
 Source: youtube.com
Source: youtube.com
Cyclohexane Chair Conformation To Double Newman Projection Youtube Chair Conformation and Ring Flips - YouTube. Additionally either bond-line or Newman formulas reveal that the two hydrogen atoms at each CH2 are not the same. Because 2-3 and 5-6 are side by side while 1-2 and 4-5 are not side by side even though both pairs are parallel. Bicyclic compounds that have bridgehead double bonds within larger rings are more stable and can be isolated. Can a chair conformation include a double bond on a ring. The chair structure of cyclohexane is considered to be the perfect conformation.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
Analysing Reaction With Chair Conformation Of Substituted Cyclohexane Chemistry Stack Exchange Take a molecule i made up rn 1-hydroxy-3-methyl-5-cyclohexene this drawn is in the shape of a ring has a hydroxy at the top and a methane at the 3rd spot and a double bond on the fifth and 6th carbon could i draw this on a chair conformation i assume not because of the pi bond but i am not sure. Even if you are explicitly given the 2-D cyclohexane you should convert it into the 3-D chair prior to solving the E2 reaction. The boat and chair conformations are interconvertable by passing through some very unstable high energy structures called the half-chair and the twist-boat conformations. A fair agreement is found. There will be the 2 rm2 2 possibilities in which the two chair forms of the decalin can be connected. Since there are two equivalent chair conformations of cyclohexane in rapid equilibrium all twelve hydrogens have 50 equatorial and 50 axial character.
 Source: researchgate.net
Source: researchgate.net
Conformation And Dihedral Angles Of Cyclohexene As Part Of The Tetralin Download Scientific Diagram Although the hydrocarbon cyclohexane is typically drawn as if it were flat in reality the structure is not flat at all. One is the cis-decalin in which the hydrogens present at the ring junction are on the same side and the other is the trans-decalin in which the ring-junction hydrogens are on the. Bicyclic compounds that have bridgehead double bonds within larger rings are more stable and can be isolated. This is similar to the double-bond twisting that would occur in trans-cyclohex-ene. And now the stabilities. For each chair conformer add the energy of all the groups on axial position.
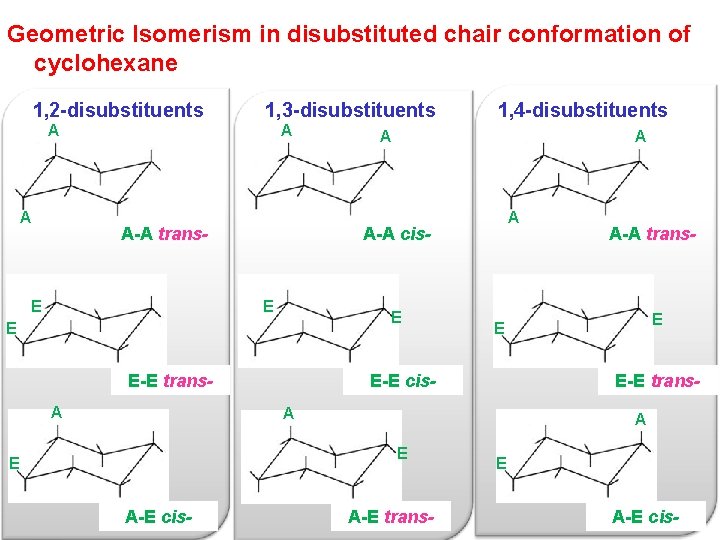 Source: slidetodoc.com
Source: slidetodoc.com
Chapter 4 Alkanes Alkenes And Alkynes Nomenclature Conformational Draw the second chair conformation ring-flip-check this post if not sure. Substituent Y is now axial to ring B and equatorial to ring A. And now the stabilities. The strain energies of cyclenes with one double bond in the ring ranging from C 6 to C 9 have been calculated as a function of various geometric parameters. Cis to rings A and B. All bond angles are 1095o and all C-H bonds are perfectly staggered Cyclohexane.
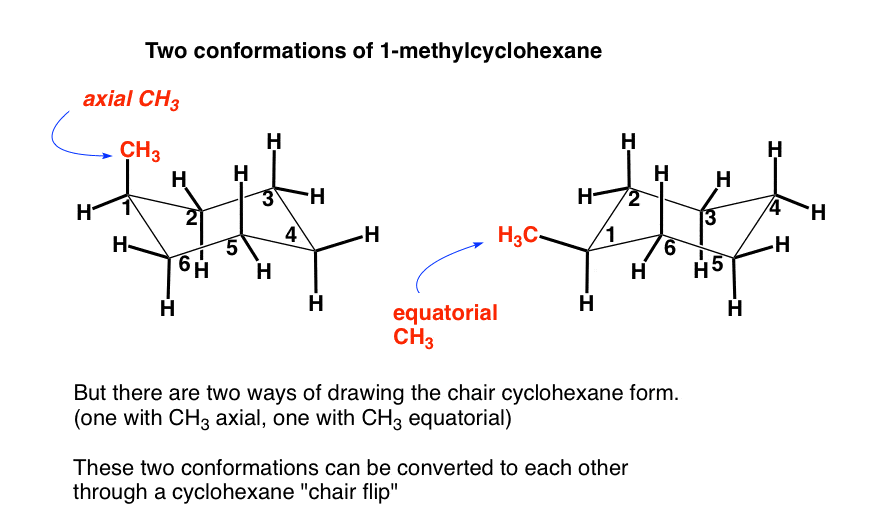 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
The Cyclohexane Chair Flip Master Organic Chemistry The chair structure of cyclohexane is considered to be the perfect conformation. The 6 rm6 6 membered rings of decalin similar form of cyclohexane which are estimated to be more stable in the chair form. Take a molecule i made up rn 1-hydroxy-3-methyl-5-cyclohexene this drawn is in the shape of a ring has a hydroxy at the top and a methane at the 3rd spot and a double bond on the fifth and 6th carbon could i draw this on a chair conformation i assume not because of the pi bond but i am not sure. Chair Conformation and Ring Flips - YouTube. The conformations of minimum energy are discussed and the energies compared with available spectroscopic and thermodynamic data. Substituent Y is now axial to ring B and equatorial to ring A.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
Analysing Reaction With Chair Conformation Of Substituted Cyclohexane Chemistry Stack Exchange There will be the 2 rm2 2 possibilities in which the two chair forms of the decalin can be connected. With the double bond in α-position of the keto function the resulting delocalisation and hence flattening of the adjacent atoms in the ring prohibit the occurrence of a chair conformation. Number the ring and draw any chair conformation of the compound. Even if you are explicitly given the 2-D cyclohexane you should convert it into the 3-D chair prior to solving the E2 reaction. The chair diagram is saying that the head of the chair is 1 and the legs of the chair is 4. With no torsional strain and no angle strain cyclohexane is the most stable of all the small rings of.
 Source: sciencedirect.com
Source: sciencedirect.com
Ring Flipping An Overview Sciencedirect Topics Cyclohexane adopts a CHAIR CONFORMATION that has no ring strain and no angle strain. I Chair conformations are generally more stable than other possibilities. Most of the time the structure exists in what is called the chair conformation. With no torsional strain and no angle strain cyclohexane is the most stable of all the small rings of. The chair diagram is saying that the head of the chair is 1 and the legs of the chair is 4. This is similar to the double-bond twisting that would occur in trans-cyclohex-ene.

Chemical Forums How Do I Tell Which Are Axial And Which Are Equatorial Cyclohexane and the Chair Structure. This conformation is called the chair because it looks sort of like a reclining lounge chair as shown here. Number the ring and draw any chair conformation of the compound. A fair agreement is found. The C-C-C bonds are very close to 1095 o so it is almost free of angle strain. Ii Substituents on chair conformers prefer to occupy equatorial positions due to the increased steric hindrance of axial locations.
 Source: researchgate.net
Source: researchgate.net
Chemical Structures Of Benzene Top And Cyclohexane Bottom Benzene Download Scientific Diagram I Chair conformations are generally more stable than other possibilities. I Chair conformations are generally more stable than other possibilities. Like the corresponding trans-cycloalkenes bicyclic compounds containing bridgehead double bonds solely within small rings are too unstable to isolate. This conformation is called the chair because it looks sort of like a reclining lounge chair as shown here. Start with a chair cyclohexane. This is similar to the double-bond twisting that would occur in trans-cyclohex-ene.
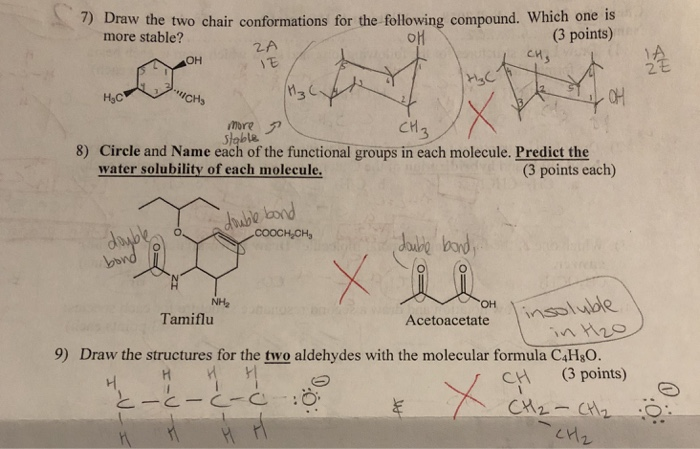 Source: chegg.com
Source: chegg.com
Solved 7 Draw The Two Chair Conformations For The Following Chegg Com Ii Substituents on chair conformers prefer to occupy equatorial positions due to the increased steric hindrance of axial locations. Substituent Y is now axial to ring B and equatorial to ring A. I Chair conformations are generally more stable than other possibilities. This is similar to the double-bond twisting that would occur in trans-cyclohex-ene. It is also a fully staggered conformation and so is free of torsional strain. A Selection of AG Values for the Change from Axial to Equatorial Orientation of Substituents for Monosubstituted Cyclohexanes.
Please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save or be able to bookmark this blog page in this website. Thank you …










