ketose chair conformation All the groups on the right side in the Fischer projection point down the groups on the left are pointing up. Draw the Hs and OH groups.
Ketose Chair Conformation, All the groups on the right side in the Fischer projection point down the groups on the left are pointing up. In the ketose shown the ketone group becomes C-2 the carbon atom next to the top Most common sugars are aldoses rather than ketoses so our discussion will focus mainly on aldoses. Ketose rearrances to mixture of aldoses which can be oxidized by Cu 2 reagents either as the open chain form or as the cyclic form.
 When Sugars Cyclize They Typically Form Furanose Or Pyranose Structures These Are Molecules With Five Membered Or Six Membered Glucose Biochemistry Chemistry From pinterest.com
When Sugars Cyclize They Typically Form Furanose Or Pyranose Structures These Are Molecules With Five Membered Or Six Membered Glucose Biochemistry Chemistry From pinterest.com
Another Article :
This video gives an elaborate explanation on how the cyclic Haworth projection is formed from the open chain Fischer projectio. Convert the Haworth to a chair conformation if needed. Remember that an aldehyde group here shown as CHO is also often written as CHO. Fischer to Haworth Projection. If the number of heavy axial groups becomes smaller when the conformation is changed to 1C4 all equatorial groups in 4C1 become axial and vice versa then it is likely that the conformation is 1C4.
The energy difference between the chair and boat forms of cyclohexane.
108B Carbohydrate Activity Author. Emil Fischer developed the Fischer Projection in order to represent these compounds. The chair conformation is customarily drawn either as 2 or as 3 which are mirror images of each other. In the chair comformation the internal bond angle at a carbon atom is 1114º very. Https D3bv2hg4q0qyg2 Cloudfront Net 2017 12 20 Ph161 E01 T02 Carbohydrates Pdf.
 Source: pinterest.com
Source: pinterest.com
Organic Chemistry Aldol Condensation Chemistry In the chair conformation look at the bond angles at the different positions of the sugars that indicate down or up on the ring structure. The twist conformation of cyclohexane. Ketose rearrances to mixture of aldoses which can be oxidized by Cu 2 reagents either as the open chain form or as the cyclic form. In the chair comformation the internal bond angle at a carbon atom is 1114º very. The most stable conformation of the cyclohexane ring is called the chair conformation. The Boat conformation is less stable than the Chair recognizable by eclipsed bonds at four Carbon atoms.
 Source: pinterest.com
Source: pinterest.com
D And L Notation For Sugars And Amino Acids Chemistry Notations Organic Chemistry Carbohydrates are really just polyhydroxyaldehydes the aldosesor polyhydroxyketones the ketoses. Bch 4053 Biochemistry I Aldose Vs Ketose Chair Conformation Chair Conformation Ochempal Aldoses vs Ketoses An aldose is a monosaccharide that has an aldehyde group at the end and a ketose is one which has a keto functional group typically residing on Carbon 2. Remember that an aldehyde group here shown as CHO is also often written as CHO. The chair conformation and the Haworth projections are alternative ways of. The twist conformation of cyclohexane. All the groups on the right side in the Fischer projection point down the groups on the left are pointing up.
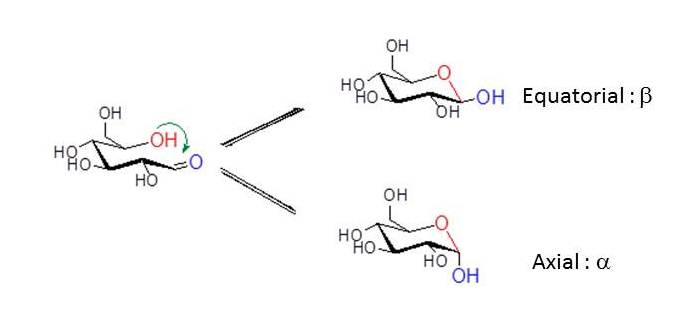 Source: glycopedia.eu
Source: glycopedia.eu
Glycopedia Fischer Projections Haworth Structures and Chair Conformers The acyclic structure of a sugar is commonly drawn as a Fischer projection. Bch 4053 Biochemistry I Aldose Vs Ketose Chair Conformation Chair Conformation Ochempal Aldoses vs Ketoses An aldose is a monosaccharide that has an aldehyde group at the end and a ketose is one which has a keto functional group typically residing on Carbon 2. Now to make a cyclic hemiacetal from your linear Fischer projection youll need to follow a few simple steps. Draw the Hs and OH groups. The energy difference between the chair and boat forms of cyclohexane. In the ketose shown the ketone group becomes C-2 the carbon atom next to the top Most common sugars are aldoses rather than ketoses so our discussion will focus mainly on aldoses.
 Source: pinterest.com
Source: pinterest.com
When Sugars Cyclize They Typically Form Furanose Or Pyranose Structures These Are Molecules With Five Membered Or Six Membered Glucose Biochemistry Chemistry To determine the chair conformation of a hexose it is generally easiest to draw it and compare it with -D-glucose where all heavy groups are equatorial and the conformation is 4C1. The 4 C 1 conformation in which C4 is above and C1 is below the plane. Carbohydrates are really just polyhydroxyaldehydes the aldosesor polyhydroxyketones the ketoses. Ring lock the ring in a single chair conformation with all of the substituents in the. Remember how to draw a Fischer projection. A ketose or keto sugar is a sugar based on a ketone rather than aldehyde functional group for its anomeric carbon.
 Source: chemistrysteps.com
Source: chemistrysteps.com
Converting Fischer Haworth And Chair Forms Of Carbohydrates Chemistry Steps And to be a ketoseit needs a ketonegroup. The cyclohexane ring 1 being large is flexible and therefore could assume an infinite number of conformations. Https D3bv2hg4q0qyg2 Cloudfront Net 2017 12 20 Ph161 E01 T02 Carbohydrates Pdf. The groups pointing up in a Haworth stay up in the chair and down stay down either axial or equatorial. 108B Carbohydrate Activity Author. Remember that an aldehyde group here shown as CHO is also often written as CHO.
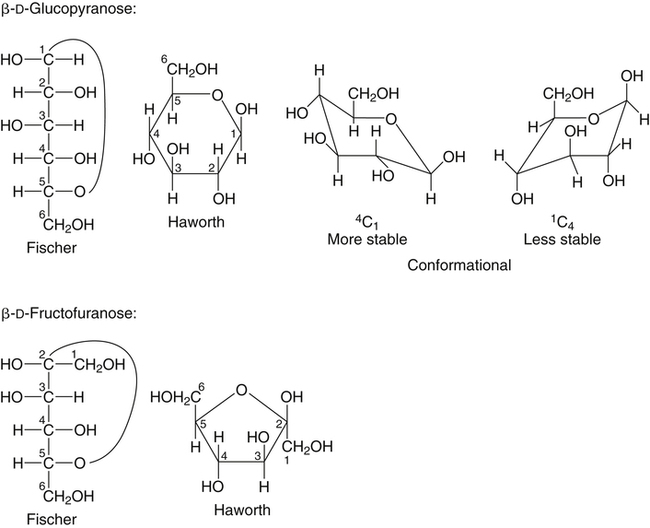 Source: basicmedicalkey.com
Source: basicmedicalkey.com
Structure Nomenclature And Properties Of Carbohydrates Basicmedical Key Remember how to draw a Fischer projection. And to be a ketoseit needs a ketonegroup. The most stable conformation of the cyclohexane ring is called the chair conformation. Fischer Projections Haworth Structures and Chair Conformers The acyclic structure of a sugar is commonly drawn as a Fischer projection. The only difference when making the polymer is that the oxygen in starch linking the units uses one equatorial and one axial bond while in cellulose it links them by two equatorial bonds. This video gives an elaborate explanation on how the cyclic Haworth projection is formed from the open chain Fischer projectio.
 Source: chemistrysteps.com
Source: chemistrysteps.com
Converting Fischer Haworth And Chair Forms Of Carbohydrates Chemistry Steps Convert the Haworth to a chair conformation if needed. Frisch spectroscopic detection of the twist-boat conformation of cyclohexane. Convert the Haworth to a chair conformation if needed. The Boat conformation is less stable than the Chair recognizable by eclipsed bonds at four Carbon atoms. These structures make it easy to show the configuration at each stereogenic center in the molecule without using wedges and dashes. Direct measurement of the free energy difference between the chair and the twist-boat.
 Source: chemistrysteps.com
Source: chemistrysteps.com
Converting Fischer Haworth And Chair Forms Of Carbohydrates Chemistry Steps D-Fructose is the most common ketose. This video gives an elaborate explanation on how the cyclic Haworth projection is formed from the open chain Fischer projectio. The only difference when making the polymer is that the oxygen in starch linking the units uses one equatorial and one axial bond while in cellulose it links them by two equatorial bonds. In the ketose shown the ketone group becomes C-2 the carbon atom next to the top Most common sugars are aldoses rather than ketoses so our discussion will focus mainly on aldoses. Draw the Hs and OH groups. Ring lock the ring in a single chair conformation with all of the substituents in the.
 Source: chempep.com
Source: chempep.com
Peptide Synthesis Custom Peptide Fmoc Amino Acids Chempep Inc In the ketose shown the ketone group becomes C-2 the carbon atom next to the top Most common sugars are aldoses rather than ketoses so our discussion will focus mainly on aldoses. So for a carbohydrate to be an aldoseit needs an aldehydegroup. The most stable conformation of the cyclohexane ring is called the chair conformation. To determine the chair conformation of a hexose it is generally easiest to draw it and compare it with -D-glucose where all heavy groups are equatorial and the conformation is 4C1. Pyranose sugars assume a chair conformation based in part on maximizing the number of large groups OH and CH 2 OH at equatorial positions which are less sterically hindered than are axial positions. The cyclohexane ring 1 being large is flexible and therefore could assume an infinite number of conformations.
 Source: slideserve.com
Source: slideserve.com
Ppt Chair Conformations Powerpoint Presentation Free Download Id 5949148 Number your atoms 1 through 5 starting from the anomeric carbon and going clockwise. The groups pointing up in a Haworth stay up in the chair and down stay down either axial or equatorial. The Boat conformation is less stable than the Chair recognizable by eclipsed bonds at four Carbon atoms. Emil Fischer developed the Fischer Projection in order to represent these compounds. Glucose the building unit of starch and cellulose is a six-membered ring that adopts a chair conformation and as a result it has axial and equatorial groups. Due to the multiple chiral centers along a linear carbon chain for carbohydrates.
 Source: chemistrysteps.com
Source: chemistrysteps.com
Converting Fischer Haworth And Chair Forms Of Carbohydrates Chemistry Steps Direct measurement of the free energy difference between the chair and the twist-boat. The energy difference between the chair and boat forms of cyclohexane. The cyclohexane ring 1 being large is flexible and therefore could assume an infinite number of conformations. The Boat conformation is less stable than the Chair recognizable by eclipsed bonds at four Carbon atoms. A ketose or keto sugar is a sugar based on a ketone rather than aldehyde functional group for its anomeric carbon. And to be a ketoseit needs a ketonegroup.
 Source: pinterest.com
Source: pinterest.com
The Diels Alder Reaction Organic Chemistry Chemistry Ochem Convert the Haworth to a chair conformation if needed. The Boat conformation is less stable than the Chair recognizable by eclipsed bonds at four Carbon atoms. If the number of heavy axial groups becomes smaller when the conformation is changed to 1C4 all equatorial groups in 4C1 become axial and vice versa then it is likely that the conformation is 1C4. This video gives an elaborate explanation on how the cyclic Haworth projection is formed from the open chain Fischer projectio. The 4 C 1 conformation in which C4 is above and C1 is below the plane. Now to make a cyclic hemiacetal from your linear Fischer projection youll need to follow a few simple steps.
 Source: twisteddnas.wordpress.com
Source: twisteddnas.wordpress.com
Chair Conformation Twisteddnas Breaking The Bond Fischer to Haworth Projection. These structures make it easy to show the configuration at each stereogenic center in the molecule without using wedges and dashes. Remember that an aldehyde group here shown as CHO is also often written as CHO. Ketose rearrances to mixture of aldoses which can be oxidized by Cu 2 reagents either as the open chain form or as the cyclic form. Https D3bv2hg4q0qyg2 Cloudfront Net 2017 12 20 Ph161 E01 T02 Carbohydrates Pdf. The 4 C 1 conformation in which C4 is above and C1 is below the plane.
 Source: youtube.com
Source: youtube.com
Carbohydrates Haworth Fischer Projections With Chair Conformations Youtube The groups pointing up in a Haworth stay up in the chair and down stay down either axial or equatorial. Fischer to Haworth Projection. In the ketose shown the ketone group becomes C-2 the carbon atom next to the top Most common sugars are aldoses rather than ketoses so our discussion will focus mainly on aldoses. The squiggly line at C-1 indicates that ring closure could create either new anomer the alpha in the down or trans conformation or the beta in the up or cis conformation. D-Fructose is the most common ketose. 108B Carbohydrate Activity Author.
 Source: researchgate.net
Source: researchgate.net
A Equatorial And Axial Directions Of The Ring Shown With E And A Download Scientific Diagram This video gives an elaborate explanation on how the cyclic Haworth projection is formed from the open chain Fischer projectio. The cyclohexane ring 1 being large is flexible and therefore could assume an infinite number of conformations. The other carbon atoms are numbered in sequence from the top. The chair conformation and the Haworth projections are alternative ways of. Bch 4053 Biochemistry I Aldose Vs Ketose Chair Conformation Chair Conformation Ochempal Aldoses vs Ketoses An aldose is a monosaccharide that has an aldehyde group at the end and a ketose is one which has a keto functional group typically residing on Carbon 2. These structures make it easy to show the configuration at each stereogenic center in the molecule without using wedges and dashes.
 Source: pinterest.com
Source: pinterest.com
Creosol Is A Chemical Compound With The Molecular Formula C8h10o2 It Is One Of The Compone How To Cook Steak Cooking Steak On Grill Cooking Classes For Kids In the chair comformation the internal bond angle at a carbon atom is 1114º very. To determine the chair conformation of a hexose it is generally easiest to draw it and compare it with -D-glucose where all heavy groups are equatorial and the conformation is 4C1. The most stable conformation of the cyclohexane ring is called the chair conformation. Fischer Projections Haworth Structures and Chair Conformers The acyclic structure of a sugar is commonly drawn as a Fischer projection. Bch 4053 Biochemistry I Aldose Vs Ketose Chair Conformation Chair Conformation Ochempal Aldoses vs Ketoses An aldose is a monosaccharide that has an aldehyde group at the end and a ketose is one which has a keto functional group typically residing on Carbon 2. A ketose or keto sugar is a sugar based on a ketone rather than aldehyde functional group for its anomeric carbon.
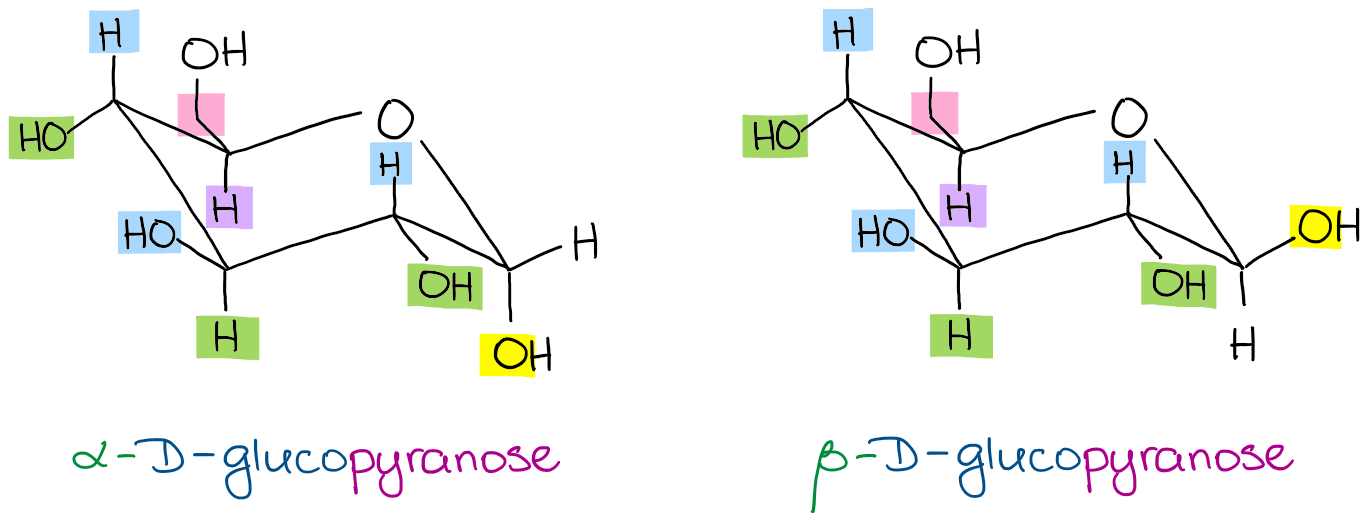 Source: organicchemistrytutor.com
Source: organicchemistrytutor.com
Converting Between Fischer Haworth And Chair Forms Of Carbohydrates Organic Chemistry Tutor Due to the multiple chiral centers along a linear carbon chain for carbohydrates. And to be a ketoseit needs a ketonegroup. Remember how to draw a Fischer projection. Emil Fischer developed the Fischer Projection in order to represent these compounds. Draw the Hs and OH groups. A ketose or keto sugar is a sugar based on a ketone rather than aldehyde functional group for its anomeric carbon.
 Source: pinterest.com
Source: pinterest.com
D And L Notation For Sugars And Amino Acids Chemistry Notations Organic Chemistry Ketose D-Monosaccharide Pyranose Triose L-Monosaccharide Glycoside Tetrose Hemiacetal Glycosidic Bond Pentose Anomeric Carbon 1. Frisch spectroscopic detection of the twist-boat conformation of cyclohexane. Ring lock the ring in a single chair conformation with all of the substituents in the. In this case the anomeric carbon is not Ci and there is no proton attached to the anomeric carbon ie it is a quaternary carbon. Direct measurement of the free energy difference between the chair and the twist-boat. Number your atoms 1 through 5 starting from the anomeric carbon and going clockwise.










