Chair conformation glucose Looking at the glucopyranose from above you now can easily draw the chair conformation for it in a general form. The unequal distribution of the two anomers is due to the fact that the -OH on the anomeric carbon of the p form is equatorial which for a chair.
Chair Conformation Glucose, The following structure is one chair conformation of beta-D-allose a sugar related to glucose. The most stable chair conformation of glucose has the C-2 C-3 and C-4 hydroxyl groups all on equatorial positions. The chair conformation is the most stable conformation of cyclohexane.
 Converting Fischer Projection To Bond Line Structure Using The R And S And Swap Method Organic Chemistry Study Organic Chemistry Chemistry Classroom From pinterest.com
Converting Fischer Projection To Bond Line Structure Using The R And S And Swap Method Organic Chemistry Study Organic Chemistry Chemistry Classroom From pinterest.com
Another Article :
This first conformation is called the chair conformation. Below you can see how to determine what goes up and what goes down on the ring. Furanose Ring systems Many sugars ketohexoses aldopentoses and in some cases even aldohexoses form five-. Similarly given the Fischer projection the chair conformation of D-glucose is. Chair And Boat Conformation Of Glucose.
The chair conformation is the most stable conformation of cyclohexane.
A chair conformation of D- glucose with equatorial and axial bonds. To draw the chair conformation of D-glucose you need to know the orientations of the axial and equatorial bonds on carbon atoms of the chair conformation. Looking at the glucopyranose from above you now can easily draw the chair conformation for it in a general form. We discuss the formation of the cyclic forms ɑ-D-glucose and β-D-glucose last time however did not pay close attention to how the Fischer forms are transformed into the cyclic anomers of glucose. The chair conformation is the most stable conformation of cyclohexane.
 Source: pinterest.com
Source: pinterest.com
The Covalent Bond Mcat Review Organic Chemistry Organic Chemistry Study Chemistry Lessons Chemistry questions and answers. This is the most stable arrangement possible. To determine the chair conformation of a hexose it is generally easiest to draw it and compare it with -D-glucose where all heavy groups are equatorial and the conformation is 4C1. The concentration of the twist-boat conformation at room temperature is very low less than 0the difference in energy between the chair and the twist-boat conformation of cyclohexane can be measured indirectly by taking the difference in activation energy for the conversion of the chair to the twist-boat. Chemistry questions and answers. A chair conformation of D- glucose with equatorial and axial bonds.
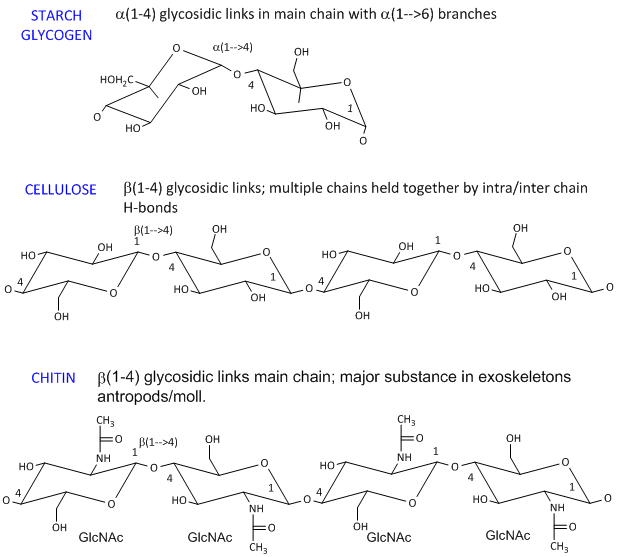 Source: pinterest.com
Source: pinterest.com
Macromolecules Biochemistry Energy Storage The most stable conformation of a glucose molecule is called the chair. We can now move arond the exterior oxygen molecules to find in which postition equatorial or axial the glucose reaches its lowest energy level. To determine the chair conformation of a hexose it is generally easiest to draw it and compare it with -D-glucose where all heavy groups are equatorial and the conformation is 4C1. Haworth projection and chair conformation of glucopyranose And just as easily you can show a chair conformations specifically for the α-D-glucopyranose and the β-D-glucopyranose. Similarly you may ask which chair conformation is more stable. The most stable conformation of a glucose molecule is called the chair.
 Source: pinterest.com
Source: pinterest.com
E1 Vs E2 Comparing The E1 And E2 Reactions Master Organic Chemistry Organic Chemistry Organic Chemistry Study Study Chemistry D-glucopyranose ring in the Haworth projection and cha. Similarly given the Fischer projection the chair conformation of D-glucose is. We can now move arond the exterior oxygen molecules to find in which postition equatorial or axial the glucose reaches its lowest energy level. If the number of heavy axial groups becomes smaller when the conformation is changed to 1C4 all equatorial groups in 4C1 become axial and vice versa then it is likely that the conformation is 1C4. The chair form of α-D-glucopyranose is The structure of β-D-glucopyranose is Prevalence of Glucose As you move around the β-glucose ring you see that all the substituents are equatorial. Overview of Chair Conformation Of Glucose.
 Source: pinterest.com
Source: pinterest.com
D And L Notation For Sugars Master Organic Chemistry Organic Chemistry Organic Chemistry Books Organic Chemistry Study It is an important subcategory of carbohydrates and is also an abundant. The most stable conformation of a glucose molecule is called the chair. This first conformation is called the chair conformation. Overview of Chair Conformation Of Glucose. The following structure is one chair conformation of beta-D-allose a sugar related to glucose. Drawing the chair form of glucose.
 Source: pinterest.com
Source: pinterest.com
Mutarotation Of Glucose Organicchemistry Ochem Orgo Ochemtutor Cheatsheet Chemistrym Organic Chemistry Organic Chemistry Reactions Chemistry Education Chemistry questions and answers. The unequal distribution of the two anomers is due to the fact that the -OH on the anomeric carbon of the p form is equatorial which for a chair. The following structure is one chair conformation of beta-D-allose a sugar related to glucose. Glucose chair conformation In the case of glucose the mutarotation gives 36 percent a 64 percent p and negligible strciight chain. This first conformation is called the chair conformation. Note 1 Of course with organic chemistry fresh in your mindyou can tell which hydroxyl groups are up and which are down in the diagram below right.
 Source: pinterest.com
Source: pinterest.com
A Values Of Axial Groups In The Cyclohexane Chair Conformation Advanced Organic Chemistry Chemistry Interactive Furanose Ring systems Many sugars ketohexoses aldopentoses and in some cases even aldohexoses form five-. Overview of Chair Conformation Of Glucose. Previous hypotheses on the conformation of a-l4-polyglucoses were actually based on the assumption that the stability of the two boat Bl and 3B forms of the a-D-glucopyranose ring was comparable to that of the chair Cl form235 In the light of more recent knowledge on conformational analysis28 it appears that the energy difference between a chair and a boat or skew form is far from being small. Below you can see how to determine what goes up and what goes down on the ring. To draw the chair conformation of D-glucose you need to know the orientations of the axial and equatorial bonds on carbon atoms of the chair conformation. Chair Conformations of Glucose.
 Source: nl.pinterest.com
Source: nl.pinterest.com
Victor The Ochem Tutor Ochemtutor Foto I Video V Instagram D Glucose Glucose Carbohydrates Note 1 Of course with organic chemistry fresh in your mindyou can tell which hydroxyl groups are up and which are down in the diagram below right. To draw the chair conformation of D-glucose you need to know the orientations of the axial and equatorial bonds on carbon atoms of the chair conformation. Glucose chair conformation In the case of glucose the mutarotation gives 36 percent a 64 percent p and negligible strciight chain. This video describes how to draw the chair conformation of D-Glucose from corresponding Haworth projection. The Fischer structure cyclizes to give the Haworth structure and this cyclic structure yields the chair conformation of glucose. Chair conformation of glucose The other anomer of glucose in contrast has its anomeric proton 31 in the axial positionIn the preferred chair conformation of glucose protons occupy all of the other axial positions leading to presumed diaxial interactions between H-l 31 and H-3 33 and between H-l 31 and H-5 35The ROESY spectrum reveals three interactions with the anomeric.
 Source: pinterest.com
Source: pinterest.com
The Diels Alder Reaction Organic Chemistry Chemistry Ochem Below you can see how to determine what goes up and what goes down on the ring. Chemistry questions and answers. Chair And Boat Conformation Of Glucose. Drawing the chair form of glucose. Previous hypotheses on the conformation of a-l4-polyglucoses were actually based on the assumption that the stability of the two boat Bl and 3B forms of the a-D-glucopyranose ring was comparable to that of the chair Cl form235 In the light of more recent knowledge on conformational analysis28 it appears that the energy difference between a chair and a boat or skew form is far from being small. D- glucopyranose also prefers an.
 Source: pinterest.com
Source: pinterest.com
A Conformational Formulas Of The Boat And Chair Forms Of The Pyranose Ring Substituents On The Rin Organic Chemistry Notes Chemistry Notes Organic Chemistry Similarly given the Fischer projection the chair conformation of D-glucose is. To determine the chair conformation of a hexose it is generally easiest to draw it and compare it with -D-glucose where all heavy groups are equatorial and the conformation is 4C1. D-Glucose can also be represented in chair form to decide the stability of alpha and beta anomersBeta anomer is more stable because all bulky groups are at. D- glucopyranose also prefers an. The three most abundant hexoses in the biological world are D-glucose D- galactose. Similarly you may ask which chair conformation is more stable.
 Source: pinterest.com
Source: pinterest.com
Pin On Tukimica Chair And Boat Conformation Of Glucose. The Fischer structure cyclizes to give the Haworth structure and this cyclic structure yields the chair conformation of glucose. Glucose is more stable than galactose simply because Glucose is less susceptible to the formation of nonspecific glycoconjugates which are molecules with at least one sugar attached to a protein or lipid. The chair conformation is the most stable conformation of cyclohexane. Chair Conformations of Glucose. The six-membered rings consisting of five carbons and one oxygen atom in the ring are collectively called pyranose and hence the ring.
 Source: cz.pinterest.com
Source: cz.pinterest.com
The Covalent Bond Organic Chemistry Study Organic Chemistry Covalent Bonding D-glucopyranose ring in the Haworth projection and cha. Haworth projection and chair conformation of glucopyranose And just as easily you can show a chair conformations specifically for the α-D-glucopyranose and the β-D-glucopyranose. The most stable conformation of a glucose molecule is called the chair. Looking at the glucopyranose from above you now can easily draw the chair conformation for it in a general form. The three most abundant hexoses in the biological world are D-glucose D- galactose. The chair conformation is the most stable conformation of cyclohexane.
 Source: pinterest.com
Source: pinterest.com
Enantiomers Vs Diastereomers Organic Chemistry Organic Chemistry Study Organic Chem We can now move arond the exterior oxygen molecules to find in which postition equatorial or axial the glucose reaches its lowest energy level. The six-membered rings consisting of five carbons and one oxygen atom in the ring are collectively called pyranose and hence the ring. Chair Conformations of Glucose - YouTube. To draw the chair conformation of D-glucose you need to know the orientations of the axial and equatorial bonds on carbon atoms of the chair conformation. Similarly given the Fischer projection the chair conformation of D-glucose is. This video describes how to draw the chair conformation of D-Glucose from corresponding Haworth projection.
 Source: pinterest.com
Source: pinterest.com
Cyclohexane Chairs Equatorial Groups Can Be Up Or Down Too Organic Chemistry Study Organic Chemistry Teaching Chemistry Previous hypotheses on the conformation of a-l4-polyglucoses were actually based on the assumption that the stability of the two boat Bl and 3B forms of the a-D-glucopyranose ring was comparable to that of the chair Cl form235 In the light of more recent knowledge on conformational analysis28 it appears that the energy difference between a chair and a boat or skew form is far from being small. It is an important subcategory of carbohydrates and is also an abundant. In todays post we will discuss the conversion between Fischer Haworth and Chair forms of carbohydrates as well the mechanism that leads to the formation of is ɑ-D-glucose and β-D-glucose. Previous hypotheses on the conformation of a-l4-polyglucoses were actually based on the assumption that the stability of the two boat Bl and 3B forms of the a-D-glucopyranose ring was comparable to that of the chair Cl form235 In the light of more recent knowledge on conformational analysis28 it appears that the energy difference between a chair and a boat or skew form is far from being small. Glucose is more stable than galactose simply because Glucose is less susceptible to the formation of nonspecific glycoconjugates which are molecules with at least one sugar attached to a protein or lipid. Chemistry questions and answers.
 Source: pinterest.com
Source: pinterest.com
Cyclohexane And Chair Flipping Intermediate Boat Conformation Organic Chemistry Video Organic Chemistry Chemistry Online School D- glucopyranose also prefers an. The Fischer structure cyclizes to give the Haworth structure and this cyclic structure yields the chair conformation of glucose. The chair form of α-D-glucopyranose is The structure of β-D-glucopyranose is Prevalence of Glucose As you move around the β-glucose ring you see that all the substituents are equatorial. Chemistry questions and answers. The six-membered rings consisting of five carbons and one oxygen atom in the ring are collectively called pyranose and hence the ring. Chair And Boat Conformation Of Glucose.
 Source: pinterest.com
Source: pinterest.com
Antiperiplanar Relationships The E2 Reaction And Cyclohexane Rings Organic Chemistry Organic Chemistry Study Chemistry Lessons Drawing the chair form of glucose. Note 1 Of course with organic chemistry fresh in your mindyou can tell which hydroxyl groups are up and which are down in the diagram below right. Chair Conformations of Glucose - YouTube. We discuss the formation of the cyclic forms ɑ-D-glucose and β-D-glucose last time however did not pay close attention to how the Fischer forms are transformed into the cyclic anomers of glucose. Looking at the glucopyranose from above you now can easily draw the chair conformation for it in a general form. D- glucopyranose also prefers an.
 Source: pinterest.com
Source: pinterest.com
Complete Collection Organic Chemistry Study Teaching Chemistry Chemistry Lessons The chair form of α-D-glucopyranose is The structure of β-D-glucopyranose is Prevalence of Glucose As you move around the β-glucose ring you see that all the substituents are equatorial. To draw the chair conformation of D-glucose you need to know the orientations of the axial and equatorial bonds on carbon atoms of the chair conformation. A chair conformation of D- glucose with equatorial and axial bonds. If the number of heavy axial groups becomes smaller when the conformation is changed to 1C4 all equatorial groups in 4C1 become axial and vice versa then it is likely that the conformation is 1C4. D-glucopyranose ring in the Haworth projection and cha. The chair form of α-D-glucopyranose is The structure of β-D-glucopyranose is Prevalence of Glucose As you move around the β-glucose ring you see that all the substituents are equatorial.
 Source: pinterest.com
Source: pinterest.com
Converting Fischer Projection To Bond Line Structure Using The R And S And Swap Method Organic Chemistry Study Organic Chemistry Chemistry Classroom It is an important subcategory of carbohydrates and is also an abundant. The six-membered rings consisting of five carbons and one oxygen atom in the ring are collectively called pyranose and hence the ring. The Fischer structure cyclizes to give the Haworth structure and this cyclic structure yields the chair conformation of glucose. To draw the chair conformation of D-glucose you need to know the orientations of the axial and equatorial bonds on carbon atoms of the chair conformation. Haworth projection and chair conformation of glucopyranose And just as easily you can show a chair conformations specifically for the α-D-glucopyranose and the β-D-glucopyranose. Note 1 Of course with organic chemistry fresh in your mindyou can tell which hydroxyl groups are up and which are down in the diagram below right.
 Source: pinterest.com
Source: pinterest.com
Organic Chemistry Educational Infographic Cyclohexane And Chair Conformation Video Organic Chemistry Chemistry Educational Infographic Similarly you may ask which chair conformation is more stable. Overview of Chair Conformation Of Glucose. We discuss the formation of the cyclic forms ɑ-D-glucose and β-D-glucose last time however did not pay close attention to how the Fischer forms are transformed into the cyclic anomers of glucose. D-Glucose can also be represented in chair form to decide the stability of alpha and beta anomersBeta anomer is more stable because all bulky groups are at. Drawing the chair form of glucose. This is the most stable arrangement possible.
Please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save or be able to bookmark this blog page in this website. Thank you …










