Chair conformation priority But before we analyze conformers lets develop the skill of viewing 3D conformers in a 2D setting and. However do I prioritize Cl over the methyl-and isopropyl-group or are the two groups more prioritized due to them being bonded to Hydrogens which take up more space than a Cl-atom.
Chair Conformation Priority, Straight chain molecules can also exhibit rotation around their single bonds. Isotopes - higher atomic mass view with lowest priority group away 1 3 2 R S 1 2 3 Cahn-Ingold-Prelog. Of cyclohexane occupy coplanar positions and when carbon atoms 3 and 6 are on opposite sides of the aircraft conformation from the D3d symmetry group is called a form of chair.
 Chiral R And S With Chair Conformers Youtube From youtube.com
Chiral R And S With Chair Conformers Youtube From youtube.com
Another Article :
If we have both a carbon group and halide does the carbon group take priority at the equatorial position. Of cyclohexane occupy coplanar positions and when carbon atoms 3 and 6 are on opposite sides of the aircraft conformation from the D3d symmetry group is called a form of chair. This is true for 1R-33-dichlorocyclohexanol. In the eclipsed conformation the three groups on the front carbon. Priority Rules at the chiral center.
For chair conformations do we always place the Halide ex Cl Br I or the carbon group CH3 ethyl etc at the equatorial position.
Direct link to Bob Of Atlantiss post In a chair conformation. From what I have read I would say that the chair conformation on the top is the most stable prioritizing the methyl- and isopropyl-group. We can think of it as two chains mirror images one of the other containing atoms 1-2-3-4 and 1-6-5-4 with opposite dihedral angles. The different spatial arrangements molecules can have are called conformers. For this reason we shall give it special attention.
 Source: study.com
Source: study.com
Build A Model Of Cyclohexanol In The Most Stable Chair Form Draw Your Model Properly Using The Chair Convention Indicate All Of The Axial And Equatorial Substituents Including Hydrogens With Study Com However do I prioritize Cl over the methyl-and isopropyl-group or are the two groups more prioritized due to them being bonded to Hydrogens which take up more space than a Cl-atom. In a chair conformation with a few exceptions only axial substituents will give rise to steric strain energy. Actually these bonds are a bit wobbly too enough for the carbons to wobble between boat and chair conformations on. The chair conformation cannot deform without changing the bond angles or lengths. An unstable conformation of cyclohexane is the boat conformation which is 71 kcalmol higher in energy than the chair form. Why is chair conformation more stable than boat conformation.
 Source: youtube.com
Source: youtube.com
Chiral R And S With Chair Conformers Youtube Priority substituents this carbon is given priority. The perfect ring Conformations of Cyclohexane Chair conformation H H H H H H H H H H H H Conformations of Cyclohexan Chair conformation Equatorial hydrogens H H H H H H H H H H H. All bond angles are 1095o and all C-H bonds are perfectly staggered Cyclohexane. Isotopes - higher atomic mass view with lowest priority group away 1 3 2 R S 1 2 3 Cahn-Ingold-Prelog. If we have both a carbon group and halide does the carbon group take priority at the equatorial position. The priority order is.
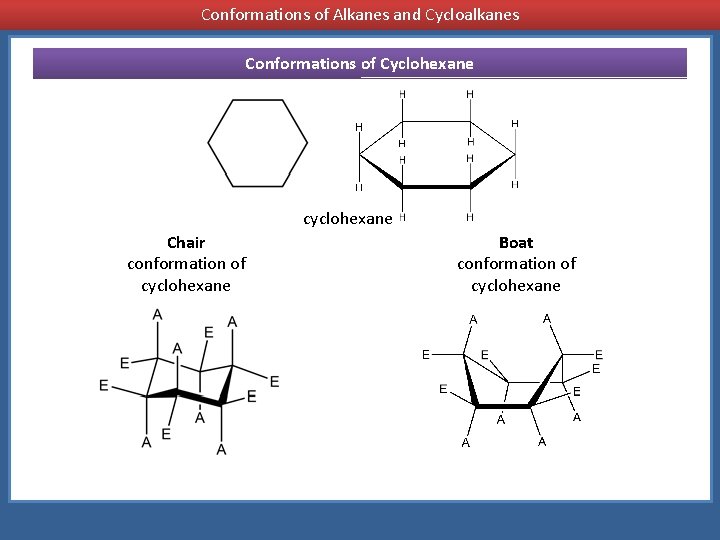 Source: slidetodoc.com
Source: slidetodoc.com
Introduction To Stereochemistry Tree Of Stereochemistry Isomers Are If we have both a carbon group and halide does the carbon group take priority at the equatorial position. The boat conformation has both steric strain due to interaction of flagpole hydrogens and torsional strain. Of cyclohexane occupy coplanar positions and when carbon atoms 3 and 6 are on opposite sides of the aircraft conformation from the D3d symmetry group is called a form of chair. We have already seen that the chair conformation of cyclohexane is the most stable one and that it is the predominant conformation of the molecules in a sample of cyclohexane. For this reason we shall give it special attention. However do I prioritize Cl over the methyl-and isopropyl-group or are the two groups more prioritized due to them being bonded to Hydrogens which take up more space than a Cl-atom.
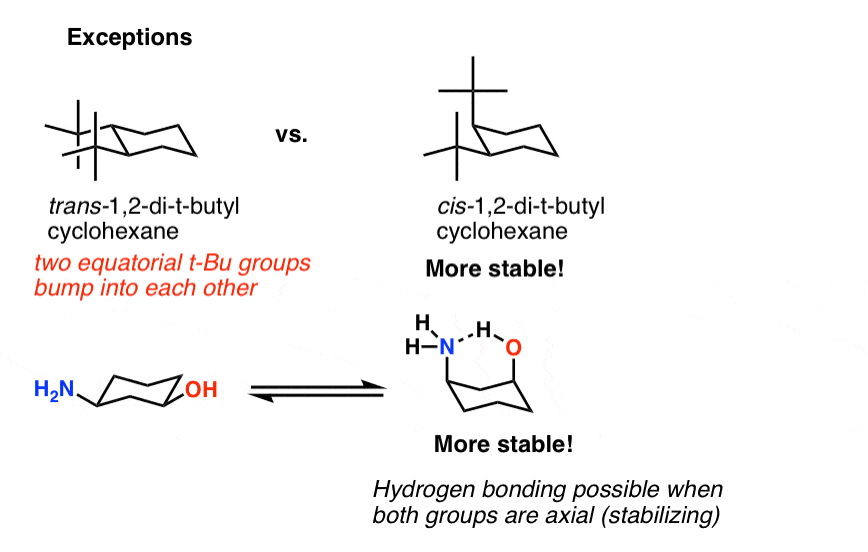 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Cyclohexane Chair Conformation Stability Which One Is Lower Energy If atoms same go to next atom 3. Cyclohexane adopts a CHAIR CONFORMATION that has no ring strain and no angle strain. Always place the largesthighest priority group in the equatorial position. The chair conformation cannot deform without changing the bond angles or lengths. We can think of it as two chains mirror images one of the other containing atoms 1-2-3-4 and 1-6-5-4 with opposite dihedral angles. The distance from atom 1 to atom 4 depends on the absolute value of the dihedral angle.
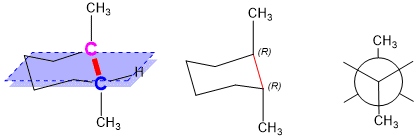 Source: studyorgo.com
Source: studyorgo.com
Stereochemistry Organic Chemistry Help The distance from atom 1 to atom 4 depends on the absolute value of the dihedral angle. But before we analyze conformers lets develop the skill of viewing 3D conformers in a 2D setting and. The different spatial arrangements molecules can have are called conformers. The chair conformation cannot deform without changing the bond angles or lengths. An unstable conformation of cyclohexane is the boat conformation which is 71 kcalmol higher in energy than the chair form. At room temperature molecules rotate and vibrate.
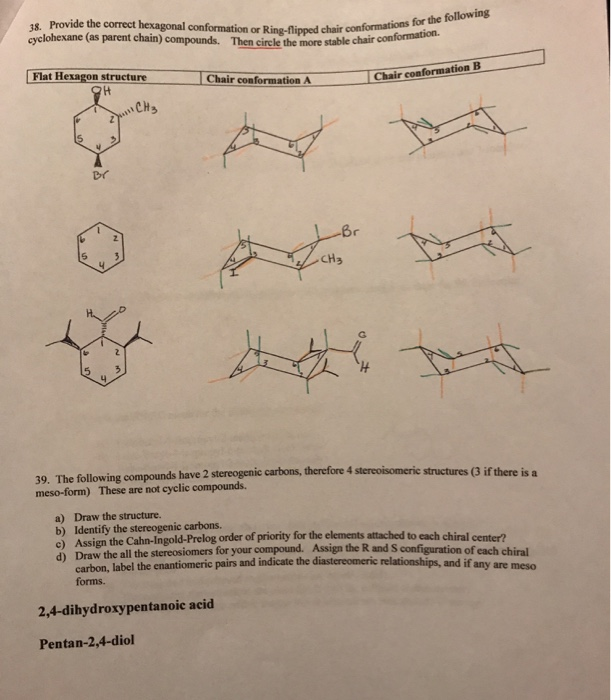 Source: chegg.com
Source: chegg.com
Solved 18 Provide The Correct Hexagonal Conformation Or Chegg Com The same term applies to similar conformations of six-member saturated ring analogous structures. If atoms same go to next atom 3. Hence configuration is R. However keep in mind that the geometry of the cyclohexane needs to be. Draw the second chair conformation ring-flip-check this post if not sure. In the first conformer we have two chlorines in axial positions so the total steric strain is.
 Source: teachthemechanism.com
Source: teachthemechanism.com
Rules Of Thumb Rots For Chair Conformations And Substituent Stability Teach The Mechanism Always place the largesthighest priority group in the equatorial position. Draw the second chair conformation ring-flip-check this post if not sure. The chair conformation is perfectly staggered about all C-C-C bonds and therefore there is no torsional strain. If we have both a carbon group and halide does the carbon group take priority at the equatorial position. Why is chair conformation more stable than boat conformation. The boat conformation has both steric strain due to interaction of flagpole hydrogens and torsional strain.
 Source: teachthemechanism.com
Source: teachthemechanism.com
Rules Of Thumb Rots For Chair Conformations And Substituent Stability Teach The Mechanism We have already seen that the chair conformation of cyclohexane is the most stable one and that it is the predominant conformation of the molecules in a sample of cyclohexane. Stereoisomerism and Cyclohexane Chairs. All bond angles are 1095o and all C-H bonds are perfectly staggered Cyclohexane. Number the ring and draw any chair conformation of the compound. The chair and boat conformations of cyclohexane are virtually angle strain free. At chiral carbon Cc.
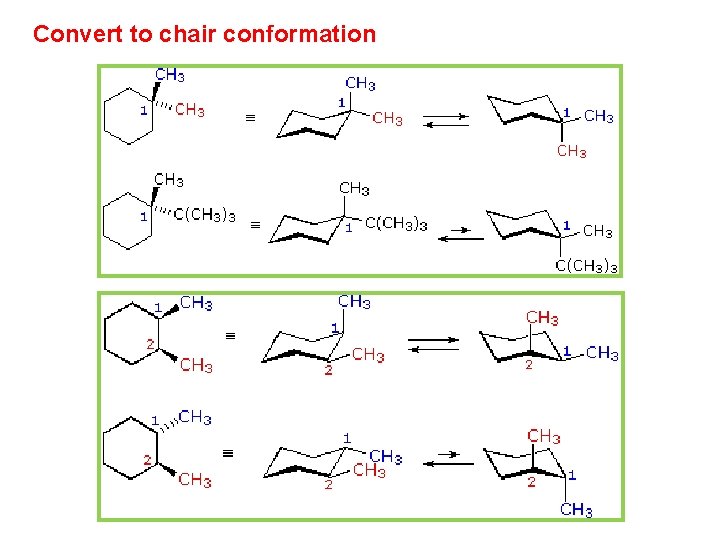 Source: slidetodoc.com
Source: slidetodoc.com
Chapter 4 Alkanes Alkenes And Alkynes Nomenclature Conformational However keep in mind that the geometry of the cyclohexane needs to be. The chair conformation that puts the larger groups in the equatorial position is energetically preferred. OH CHisoPr CH2 H. Actually these bonds are a bit wobbly too enough for the carbons to wobble between boat and chair conformations on. For each chair conformer add the energy of all the groups on axial position. And now the stabilities.
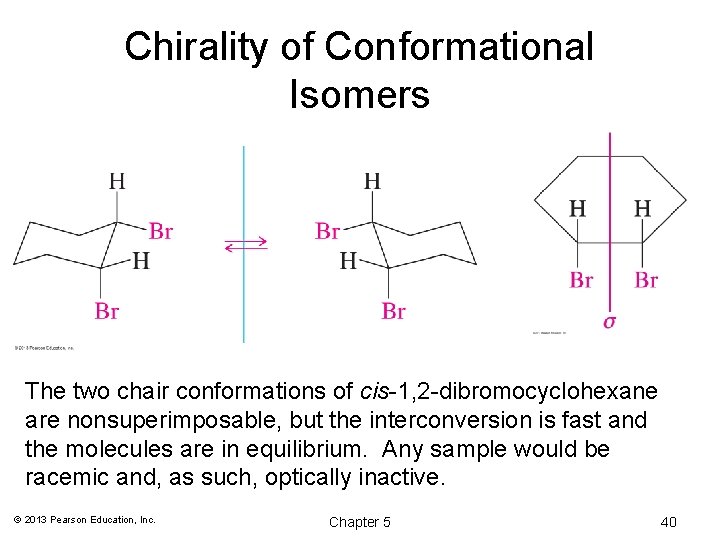 Source: slidetodoc.com
Source: slidetodoc.com
Stereochemistry Refers To The 3 Dimensional Properties And Of cyclohexane occupy coplanar positions and when carbon atoms 3 and 6 are on opposite sides of the aircraft conformation from the D3d symmetry group is called a form of chair. Isotopes - higher atomic mass view with lowest priority group away 1 3 2 R S 1 2 3 Cahn-Ingold-Prelog. Why is chair conformation more stable than boat conformation. The boat conformation has both steric strain due to interaction of flagpole hydrogens and torsional strain. The distance from atom 1 to atom 4 depends on the absolute value of the dihedral angle. If we have both a carbon group and halide does the carbon group take priority at the equatorial position.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Cyclohexane Chair Conformation Stability Which One Is Lower Energy Atomic number priority 1234 largest atomic number first 2. The six-membered ring is the most common ring found among natures organic molecules. At room temperature molecules rotate and vibrate. At chiral carbon Cc. But before we analyze conformers lets develop the skill of viewing 3D conformers in a 2D setting and. This is true for 1R-33-dichlorocyclohexanol.
 Source: youtube.com
Source: youtube.com
Chair Conformations Examples Youtube CHOH isoPr CH2 H. But before we analyze conformers lets develop the skill of viewing 3D conformers in a 2D setting and. The tert-butyl group needs to be placed in the equatorial bond as it is the lowest energy or highest stability conformation. Of cyclohexane occupy coplanar positions and when carbon atoms 3 and 6 are on opposite sides of the aircraft conformation from the D3d symmetry group is called a form of chair. The chair conformation is perfectly staggered about all C-C-C bonds and therefore there is no torsional strain. Isotopes - higher atomic mass view with lowest priority group away 1 3 2 R S 1 2 3 Cahn-Ingold-Prelog.
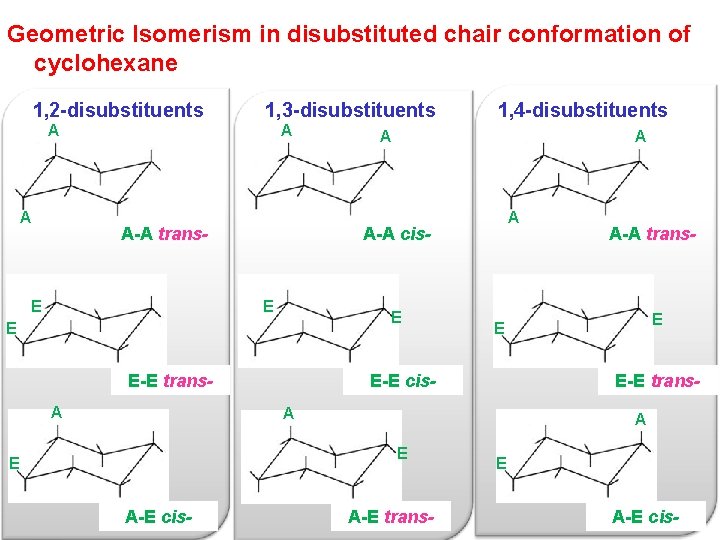 Source: slidetodoc.com
Source: slidetodoc.com
Chapter 4 Alkanes Alkenes And Alkynes Nomenclature Conformational Atomic number priority 1234 largest atomic number first 2. Orient molecule so that the lowest priority group group 4 is. The boat conformation has both steric strain due to interaction of flagpole hydrogens and torsional strain. The perfect ring Conformations of Cyclohexane Chair conformation H H H H H H H H H H H H Conformations of Cyclohexan Chair conformation Equatorial hydrogens H H H H H H H H H H H. And now the stabilities. We can think of it as two chains mirror images one of the other containing atoms 1-2-3-4 and 1-6-5-4 with opposite dihedral angles.
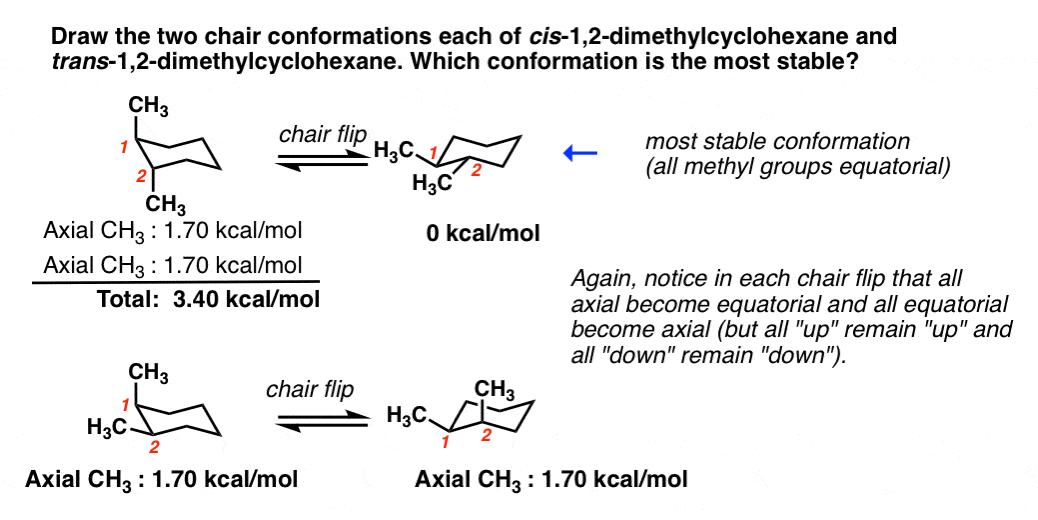 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Cyclohexane Chair Conformation Stability Which One Is Lower Energy For chair conformations do we always place the Halide ex Cl Br I or the carbon group CH3 ethyl etc at the equatorial position. Actually these bonds are a bit wobbly too enough for the carbons to wobble between boat and chair conformations on. Always place the largesthighest priority group in the equatorial position. For each chair conformer add the energy of all the groups on axial position. The different spatial arrangements molecules can have are called conformers. We can think of it as two chains mirror images one of the other containing atoms 1-2-3-4 and 1-6-5-4 with opposite dihedral angles.
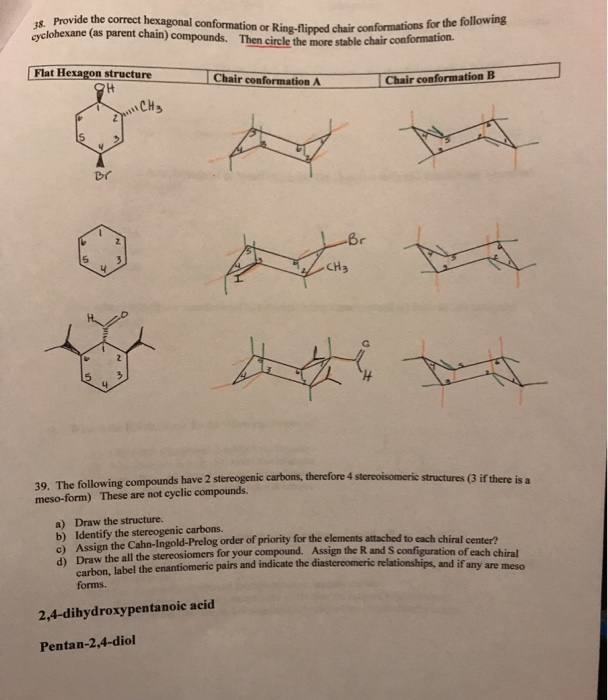 Source: chegg.com
Source: chegg.com
Solved 38 Provide The Correct Hexagonal Conformation Or Chegg Com Br OH H H H H Viewer Br H OH HH front. The most stable and most important conformation of cyclohexane is the chair conformation shown below. Boat chair The boat and chair conformations are interconvertable by passing through. Chair conformation of cyclohexane is more stable than boat form because in chair conformaion the C-H bonds are equally axial and equatorial ie out of twelve C-H bonds six are axial and six are equatorial and each carbon has one axial and one equatorial C-H bond. The tert-butyl group needs to be placed in the equatorial bond as it is the lowest energy or highest stability conformation. The different spatial arrangements molecules can have are called conformers.
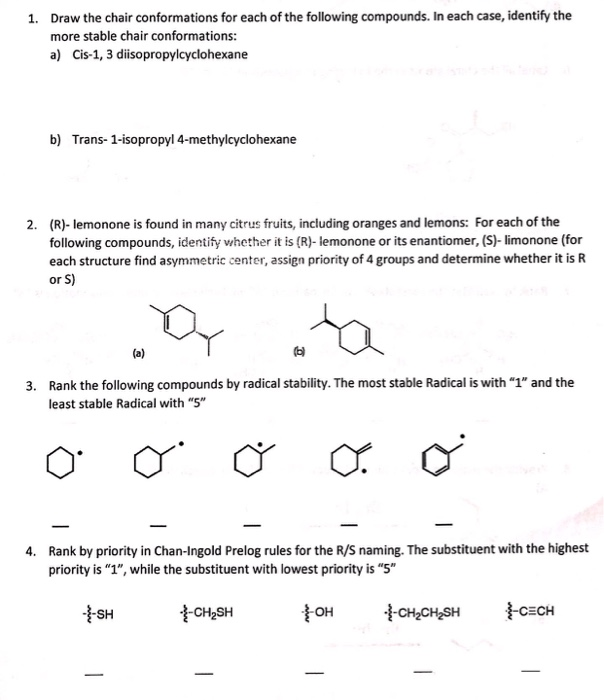 Source: chegg.com
Source: chegg.com
Solved Draw The Chair Conformations For Each Of The Chegg Com The lowest priority group here it is H is going away from the viewer and eyesight moves in a clockwise direction. From what I have read I would say that the chair conformation on the top is the most stable prioritizing the methyl- and isopropyl-group. The distance from atom 1 to atom 4 depends on the absolute value of the dihedral angle. Br OH H H H H Viewer Br H OH HH front. The chair with all bonds staggered and no torsional strain is the lowest-energy conformation of cyclohexane. Stereoisomerism and Cyclohexane Chairs.
 Source: youtube.com
Source: youtube.com
How To Draw Cyclohexane Chair Conformations And Ring Flips Youtube An unstable conformation of cyclohexane is the boat conformation which is 71 kcalmol higher in energy than the chair form. If we have both a carbon group and halide does the carbon group take priority at the equatorial position. We have already seen that the chair conformation of cyclohexane is the most stable one and that it is the predominant conformation of the molecules in a sample of cyclohexane. The chair conformation cannot deform without changing the bond angles or lengths. OH CHisoPr CH2 H. Hence configuration is R.
 Source: youtube.com
Source: youtube.com
Chiral R And S With Chair Conformers Youtube Draw the second chair conformation ring-flip-check this post if not sure. The chair conformation that puts the larger groups in the equatorial position is energetically preferred. The twist-boat relieves some of the steric strain and torsional strain encountered in the boat conformation so it is slightly lower in energy than the boat. At chiral carbon Cc. Always place the largesthighest priority group in the equatorial position. The chair conformation is perfectly staggered about all C-C-C bonds and therefore there is no torsional strain.
Please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save or be able to bookmark this blog page in this website. Thank you …










