Chair conformation r and s There is a severe crowding among the atoms in axial positions on the same side of the ring. No angle strain - angles must be 1095 2.
Chair Conformation R And S, Other chair questions they could ask would be to correctly position axial and equitorial groups or to determine which chair is the most stable. Notice how the axial-methyl group is thrown closely together with the axial hydrogens in carbons 3 and 5. Hence configuration is S.
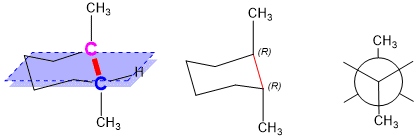 Stereochemistry Organic Chemistry Help From studyorgo.com
Stereochemistry Organic Chemistry Help From studyorgo.com
Another Article :
Given your value in a calculate the percent of the chair indicated as B presented in an equilibrium mixture of the conformers at 25ºC. RS convention also known as Cahn-Ingold-Prelog convention or more conveniently CIP convention is the universally recognized method used to specify absolute configuration at chiral centers in organic molecules. The lowest priority group here it is H is coming towards the viewer and eyesight moves in a clockwise direction. Isotopes - higher atomic mass view with lowest priority group away 1 3 2 R S. There is a severe crowding among the atoms in axial positions on the same side of the ring.
Hence configuration is S.
Calculate the difference in the Gibbs free energy between the second and first conformation including the algebraic sign. Isotopes - higher atomic mass view with lowest priority group away 1 3 2 R S. Looks at converting a chair conformation into a perspective skeletal structures so that R and S can be easily determined at each chiral center. Notice how the axial-methyl group is thrown closely together with the axial hydrogens in carbons 3 and 5. A The two chair conformations are equal in energy.
 Source: youtube.com
Source: youtube.com
Organic Chemistry Stereoisomerism Of Chair Conformation Youtube Boat chair The boat and chair conformations are interconvertable by passing through some very unstable high energy structures called the half-chair and the twist-boat. Atomic number priority 1234 largest atomic number first 2. Alternate your axial substituents up and down all the way around your cyclohexane. K must be given and R a constant will be provided. An unstable conformation of cyclohexane is the boat conformation which is 71 kcalmol higher in energy than the chair form. A The two chair conformations are equal in energy.
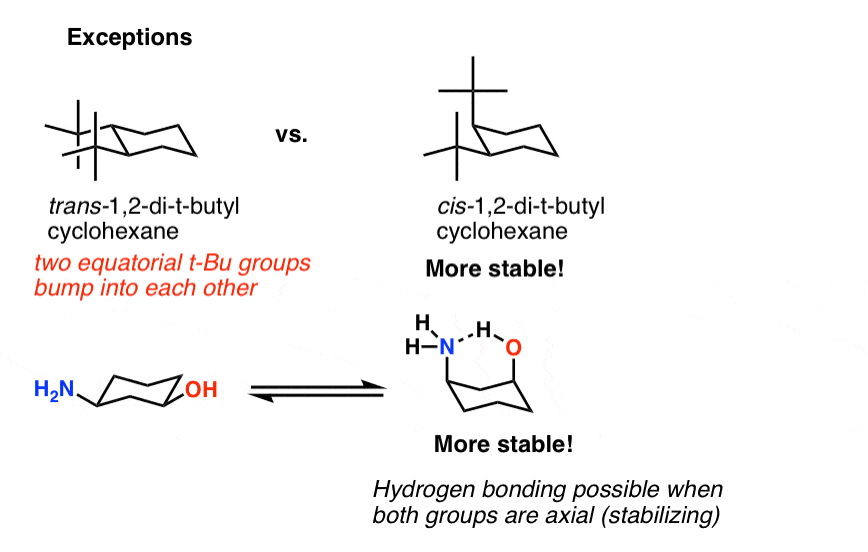 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Cyclohexane Chair Conformation Stability Which One Is Lower Energy Looks at converting a chair conformation into a perspective skeletal structures so that R and S can be easily determined at each chiral center. Alternate your axial substituents up and down all the way around your cyclohexane. Here we have a model of the cyclohexane molecule and it looks like its a flat hexagon from this perspective but it isnt really if we turn it to the side we can see this is not a planar molecule this is called the chair conformation of cyclohexane and if we stare down these two carbons well be able to see the chair conformation from a Newman projection viewpoint so now you can see that we have staggered hydrogens here we have a picture of the chair conformation. R recto right-handed S sinestro left-handed Priority Rules at the chiral center. Heres how to avoid those mistakes ace any chair conformations in your OChem exams. Given your value in a calculate the percent of the chair indicated as B presented in an equilibrium mixture of the conformers at 25ºC.
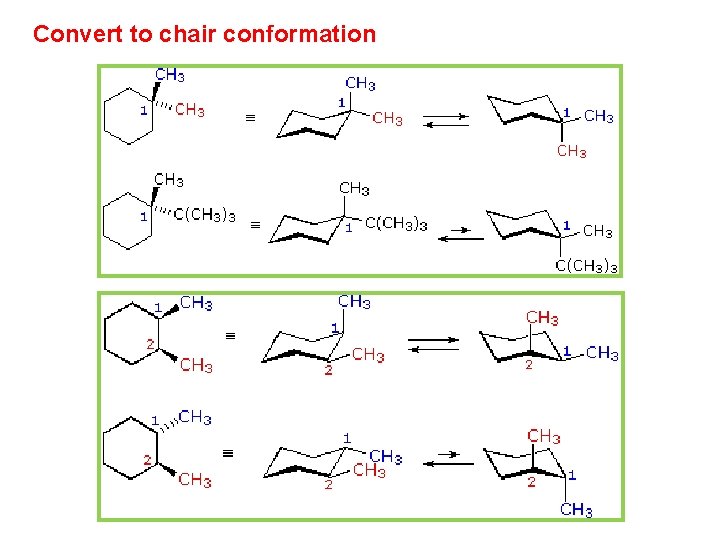 Source: slidetodoc.com
Source: slidetodoc.com
Chapter 4 Alkanes Alkenes And Alkynes Nomenclature Conformational Lets get some practice drawing it chair confirmations one way to do it is to start by drawing two parallel lines that are offset from each others let me go ahead and show you what I mean so heres heres one line and then here is another line theyre parallel to each other but theyre offset a little bit next were going to draw two horizontal dotted lines so the top horizontal dotted line is going to be on level with that top. Look at the axial-methylcyclohexane in chair conformation shown. For the two conformers of 1R-33-dichlorocyclohexanol assume the calculation is at 25C must be converted to K by 25C 273 298K Gibbs Free Energy Equation. A The two chair conformations are equal in energy. Heres how to avoid those mistakes ace any chair conformations in your OChem exams. Isotopes - higher atomic mass view with lowest priority group away 1 3 2 R S.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Cyclohexane Chair Conformation Stability Which One Is Lower Energy Cyclohexane is the chair conformation shown below. But to draw them naturally to get them flowing off your pen and onto the. R recto right-handed S sinestro left-handed Priority Rules at the chiral center. There is a severe crowding among the atoms in axial positions on the same side of the ring. Boat chair The boat and chair conformations are interconvertable by passing through some very unstable high energy structures called the half-chair and the twist-boat. No torsional strain - all adjacent C-H bonds must be staggered Cyclohexane have another conformers but The most stable conformer of cyclohexane is the chair conformation.
 Source: youtube.com
Source: youtube.com
Chiral R And S With Chair Conformers Youtube According to RS convention the absolute configuration at a chiral center is designated either R or S. Draw the two chair conformations and determine which conformation is more stable. If I need to give more information let me know. Drawing a Sugars Chair Conformation from a Haworth Structure or Fischer Projection If you have either the Haworth structure or the Fischer projection of the sugar drawing the chair conformation of the sugar is easy as long as you remember the orientations of the axial and equatorial bonds on each carbon atom of the chair conformation. Cyclohexane Cyclohexane is considered to have ZERO ring strain in its optimal conformation Called chair conformation. An unstable conformation of cyclohexane is the boat conformation which is 71 kcalmol higher in energy than the chair form.
 Source: youtube.com
Source: youtube.com
R S Configuration For Chair Form Of Cyclohexane Chiral Carbon Youtube An unstable conformation of cyclohexane is the boat conformation which is 71 kcalmol higher in energy than the chair form. Given your value in a calculate the percent of the chair indicated as B presented in an equilibrium mixture of the conformers at 25ºC. K must be given and R a constant will be provided. Isotopes - higher atomic mass view with lowest priority group away 1 3 2 R S. Hence configuration is S. RS convention also known as Cahn-Ingold-Prelog convention or more conveniently CIP convention is the universally recognized method used to specify absolute configuration at chiral centers in organic molecules.
 Source: youtube.com
Source: youtube.com
Determining R And S Practice Youtube There is a severe crowding among the atoms in axial positions on the same side of the ring. C C C C O CC is CO is O 4. Other chair questions they could ask would be to correctly position axial and equitorial groups or to determine which chair is the most stable. Boat chair The boat and chair conformations are interconvertable by passing through some very unstable high energy structures called the half-chair and the twist-boat. CHOH isoPr CH2 H. K must be given and R a constant will be provided.
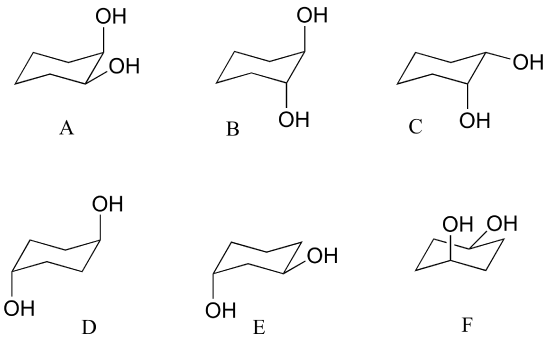 Source: chem.libretexts.org
Source: chem.libretexts.org
3 P Problems For Chapter 3 Chemistry Libretexts There is a severe crowding among the atoms in axial positions on the same side of the ring. Calculate the difference in the Gibbs free energy between the second and first conformation including the algebraic sign. Drawing a Sugars Chair Conformation from a Haworth Structure or Fischer Projection If you have either the Haworth structure or the Fischer projection of the sugar drawing the chair conformation of the sugar is easy as long as you remember the orientations of the axial and equatorial bonds on each carbon atom of the chair conformation. Boat chair The boat and chair conformations are interconvertable by passing through some very unstable high energy structures called the half-chair and the twist-boat. Atomic number priority 1234 largest atomic number first 2. There is a severe crowding among the atoms in axial positions on the same side of the ring.
 Source: studyorgo.com
Source: studyorgo.com
Stereochemistry Organic Chemistry Help The lowest priority group here it is H is coming towards the viewer and eyesight moves in a clockwise direction. Look at the axial-methylcyclohexane in chair conformation shown. Cyclohexane Cyclohexane is considered to have ZERO ring strain in its optimal conformation Called chair conformation. Draw the two chair conformations and determine which conformation is more stable. Cyclohexane is the chair conformation shown below. Given your value in a calculate the percent of the chair indicated as B presented in an equilibrium mixture of the conformers at 25ºC.
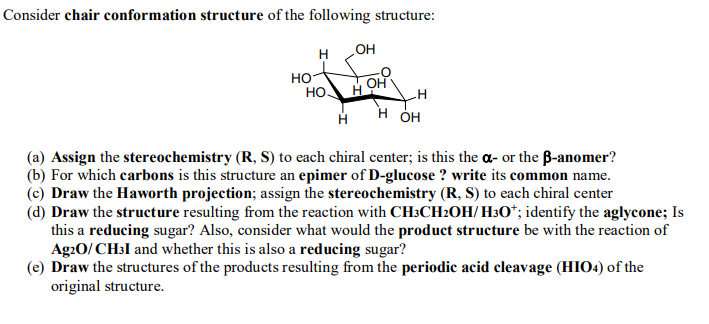 Source: chegg.com
Source: chegg.com
Solved Consider Chair Conformation Structure Of The Chegg Com Looks at converting a chair conformation into a perspective skeletal structures so that R and S can be easily determined at each chiral center. There is a severe crowding among the atoms in axial positions on the same side of the ring. But to draw them naturally to get them flowing off your pen and onto the. C C C C O CC is CO is O 4. Cyclohexane is the chair conformation shown below. If atoms same go to next atom 3.
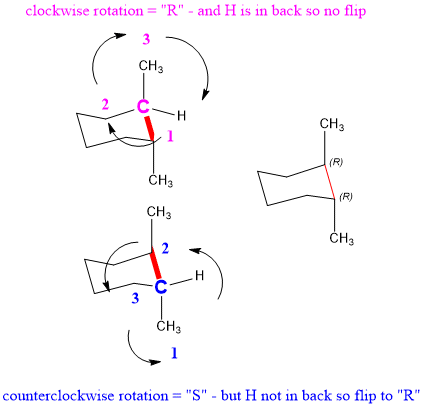 Source: studyorgo.com
Source: studyorgo.com
Stereochemistry Organic Chemistry Help At chiral carbon Cc. Cyclohexane Cyclohexane is considered to have ZERO ring strain in its optimal conformation Called chair conformation. C C C C O CC is CO is O 4. Notice how the axial-methyl group is thrown closely together with the axial hydrogens in carbons 3 and 5. Look at the axial-methylcyclohexane in chair conformation shown. For the two conformers of 1R-33-dichlorocyclohexanol assume the calculation is at 25C must be converted to K by 25C 273 298K Gibbs Free Energy Equation.
 Source: researchgate.net
Source: researchgate.net
Configuration And Preferred Half Chair Conformation Of The Two Download Scientific Diagram Once youve mastered the art of drawing chair conformations its time to stick some axial and equatorial substituents on those beautiful chairsIn marking thousands of exam papers theres 2 mistakes that Ive seen over and over again. Isotopes - higher atomic mass view with lowest priority group away 1 3 2 R S. Drawing a Sugars Chair Conformation from a Haworth Structure or Fischer Projection If you have either the Haworth structure or the Fischer projection of the sugar drawing the chair conformation of the sugar is easy as long as you remember the orientations of the axial and equatorial bonds on each carbon atom of the chair conformation. A The two chair conformations are equal in energy. Cyclohexane is the chair conformation shown below. Heres how to avoid those mistakes ace any chair conformations in your OChem exams.
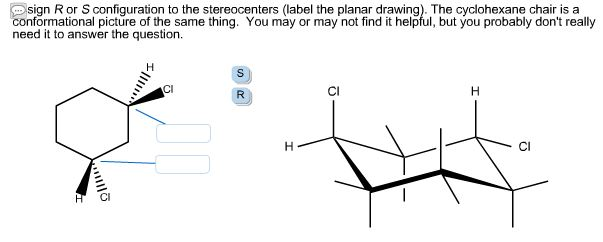 Source: chegg.com
Source: chegg.com
Solved Assign R Or S Configuration To The Stereocenters Chegg Com E The lower energy chair conformation contains two equatorial ethyl groups. The lowest priority group here it is H is coming towards the viewer and eyesight moves in a clockwise direction. Alternate your axial substituents up and down all the way around your cyclohexane. Other chair questions they could ask would be to correctly position axial and equitorial groups or to determine which chair is the most stable. Cyclohexane Cyclohexane is considered to have ZERO ring strain in its optimal conformation Called chair conformation. Every carbon on the chair conformation has.
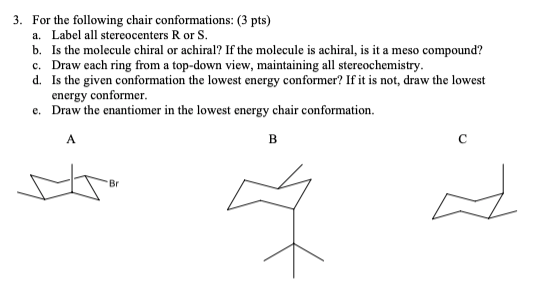
Solved For The Following Chair Conformations 3 Pts A Chegg Com Alternate your axial substituents up and down all the way around your cyclohexane. D The lower energy chair conformation contains two axial ethyl groups. CHOH isoPr CH2 H. B The higher energy chair conformation contains two axial ethyl groups. Notice how the axial-methyl group is thrown closely together with the axial hydrogens in carbons 3 and 5. R recto right-handed S sinestro left-handed Priority Rules at the chiral center.
 Source: youtube.com
Source: youtube.com
How To Draw Cyclohexane Chair Conformations And Ring Flips Youtube D The lower energy chair conformation contains two axial ethyl groups. C C C C O CC is CO is O 4. Boat chair The boat and chair conformations are interconvertable by passing through some very unstable high energy structures called the half-chair and the twist-boat. If I need to give more information let me know. Other chair questions they could ask would be to correctly position axial and equitorial groups or to determine which chair is the most stable. RS convention also known as Cahn-Ingold-Prelog convention or more conveniently CIP convention is the universally recognized method used to specify absolute configuration at chiral centers in organic molecules.
 Source: youtube.com
Source: youtube.com
Chiral R And S With Chair Conformers Youtube An unstable conformation of cyclohexane is the boat conformation which is 71 kcalmol higher in energy than the chair form. Here we have a model of the cyclohexane molecule and it looks like its a flat hexagon from this perspective but it isnt really if we turn it to the side we can see this is not a planar molecule this is called the chair conformation of cyclohexane and if we stare down these two carbons well be able to see the chair conformation from a Newman projection viewpoint so now you can see that we have staggered hydrogens here we have a picture of the chair conformation. Looks at converting a chair conformation into a perspective skeletal structures so that R and S can be easily determined at each chiral center. Calculate the difference in the Gibbs free energy between the second and first conformation including the algebraic sign. Draw the two chair conformations and determine which conformation is more stable. The chair conformation has alternating axial up axial down so once you have that single axial substituent move on to.
 Source: youtube.com
Source: youtube.com
Chair Conformation And Ring Flips Youtube There is a severe crowding among the atoms in axial positions on the same side of the ring. Every carbon on the chair conformation has. Alternate your axial substituents up and down all the way around your cyclohexane. According to RS convention the absolute configuration at a chiral center is designated either R or S. D The lower energy chair conformation contains two axial ethyl groups. No angle strain - angles must be 1095 2.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
A Simple Trick The R S Toggle Here we have a model of the cyclohexane molecule and it looks like its a flat hexagon from this perspective but it isnt really if we turn it to the side we can see this is not a planar molecule this is called the chair conformation of cyclohexane and if we stare down these two carbons well be able to see the chair conformation from a Newman projection viewpoint so now you can see that we have staggered hydrogens here we have a picture of the chair conformation. B The higher energy chair conformation contains two axial ethyl groups. Here we have a model of the cyclohexane molecule and it looks like its a flat hexagon from this perspective but it isnt really if we turn it to the side we can see this is not a planar molecule this is called the chair conformation of cyclohexane and if we stare down these two carbons well be able to see the chair conformation from a Newman projection viewpoint so now you can see that we have staggered hydrogens here we have a picture of the chair conformation. ΔG -RT ln Keq R 0002kcalKmol ΔG -RT ln Keq-10kcalmol -0002kcalKmol 298K lnKeq. Boat chair The boat and chair conformations are interconvertable by passing through some very unstable high energy structures called the half-chair and the twist-boat. Once youve mastered the art of drawing chair conformations its time to stick some axial and equatorial substituents on those beautiful chairsIn marking thousands of exam papers theres 2 mistakes that Ive seen over and over again.
Please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark or be able to bookmark this blog page in this website. Thank you …










