Chair conformation structure The Stereochemistry of the Anomeric Carbon the α-form or the β-form Converting Haworth to Chair. The chair conformation has alternating axial up axial down so once you have that single axial substituent move on to.
Chair Conformation Structure, Although it is more stable the chair conformation is much more rigid than the boat conformation. The chair structure consists of a six-membered ring where every C-C bond exists in a staggered conformation. Drawing a Sugars Chair Conformation from a Haworth Structure or Fischer Projection If you have either the Haworth structure or the Fischer projection of the sugar drawing the chair conformation of the sugar is easy as long as you remember the orientations of the axial and equatorial bonds on each carbon atom of the chair conformation.
 A Chair Conformations Of The Uronic Acid Molecules That Constitute Download Scientific Diagram From researchgate.net
A Chair Conformations Of The Uronic Acid Molecules That Constitute Download Scientific Diagram From researchgate.net
Another Article :
By flexing to a new formthe twist conformation Fig5the boat conformation can relieve some of its torsional strain and at the same time reduce the flagpole interactions. Answer this question WITHOUT drawing a chair conformationring flip. The stability is a consequence of no angle strain owing to the almost tetrahedral C-C-C bond angles and no torsional strain due to the perfectly staggered. Although the hydrocarbon cyclohexane is typically drawn as if it were flat in reality the structure is not flat at all. Draw ALL possible chair conformations for 2-tert-butylcyclohexanol then rank in order of most to least stable.
Drawing a Sugars Chair Conformation from a Haworth Structure or Fischer Projection If you have either the Haworth structure or the Fischer projection of the sugar drawing the chair conformation of the sugar is easy as long as you remember the orientations of the axial and equatorial bonds on each carbon atom of the chair conformation.
If you have not already done so you should. Although it is more stable the chair conformation is much more rigid than the boat conformation. Notice how the axial-methyl group is thrown closely together with the axial hydrogens in carbons 3 and 5. Determine if the highlighted atom will appear in the axial or equatorial position in the more stable chair conformation. This conformation of cyclohexane is called the chair conformation be-cause of its resemblance to a lawn chair.
 Source: youtube.com
Source: youtube.com
How To Draw Cyclohexane Chair Conformations And Ring Flips Youtube Drawing a Sugars Chair Conformation from a Haworth Structure or Fischer Projection If you have either the Haworth structure or the Fischer projection of the sugar drawing the chair conformation of the sugar is easy as long as you remember the orientations of the axial and equatorial bonds on each carbon atom of the chair conformation. This conformation of cyclohexane is called the chair conformation be-cause of its resemblance to a lawn chair. Explaining how A-Values are related to cyclohexane flip energy. Using the numbering scheme in the original structure shown in step 1 and the numbering scheme in the chair conformation templates drawn in step 3 fill in the substituents on the chair conformations. Calculating Flip Energy. The chair conformation has alternating axial up axial down so once you have that single axial substituent move on to.
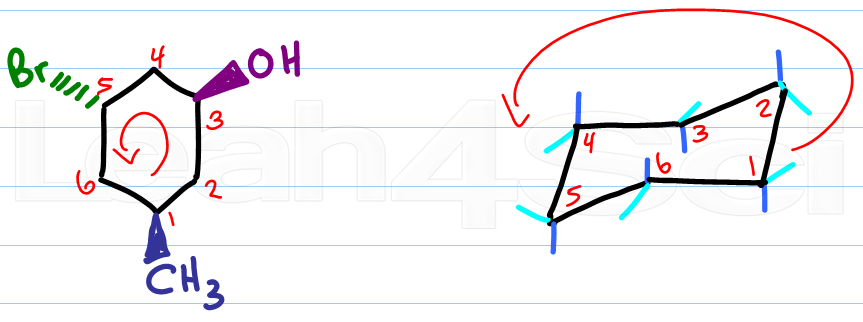 Source: leah4sci.com
Source: leah4sci.com
Drawing Chair Conformations And Ring Flips For Cyclohexane In Organic Chemistry In this post I want to go over the three most typical forms of the carbohydrates. The chair conformation being the most stable and predominant form of cyclohexane is non-planar with the headrest and footrest bonds lying above and below the planar seat bonds. Most of the time the structure exists in what is called the chair conformation. Although the hydrocarbon cyclohexane is typically drawn as if it were flat in reality the structure is not flat at all. Notice how the axial-methyl group is thrown closely together with the axial hydrogens in carbons 3 and 5. A Methylcyclohexane can exist in two different chair conformations one of which is 18 kcalmol more stable than the other ie the A value for the methyl group is 18.
 Source: youtube.com
Source: youtube.com
How To Draw Chair Conformations Of Cyclohexane Rings And Recognize Equivalent Representations Youtube Explaining how A-Values are related to cyclohexane flip energy. There are twelve possible positions on a chair structure. This is your first axial substituent. Look at the axial-methylcyclohexane in chair conformation shown. Rather the carbon skele-ton is puckered. Six of the positions exist in.
 Source: researchgate.net
Source: researchgate.net
A Chair Conformations Of The Uronic Acid Molecules That Constitute Download Scientific Diagram This is your first axial substituent. With no torsional strain and no angle strain cyclohexane is the most stable of all the small rings of the cycloalkanes. There is a severe crowding among the atoms in axial positions on the same side of the ring. Answer this question WITHOUT drawing a chair conformationring flip. Explaining how A-Values are related to cyclohexane flip energy. This conformation is called the chair because it looks sort of like a reclining lounge chair as shown here.
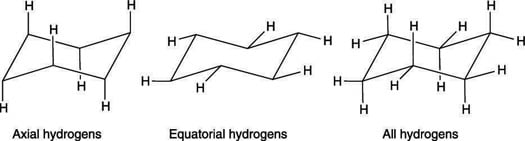 Source: dummies.com
Source: dummies.com
How To Draw The Chair Conformation Of Cyclohexane Dummies Most of the time the structure exists in what is called the chair conformation. This conformation of cyclohexane is called the chair conformation be-cause of its resemblance to a lawn chair. The definition of chair conformation is the lowest energy conformation for cyclohexane in which all bond angles are fairly close to 1095 and all hydrogen atoms are staggered. Draw ALL possible chair conformations for 2-tert-butylcyclohexanol then rank in order of most to least stable. The chair structure of cyclohexane is considered to be the perfect conformation. Actually these bonds are a bit wobbly too enough for the carbons to wobble between boat and chair conformations on occasion.
 Source: chemistrysteps.com
Source: chemistrysteps.com
Ring Flip Of Chair Conformations With Practice Problems Chemistry Steps And now the stabilities. First we have to introduce the concept of an A-value which is simply the energy difference between the equatorial most stable and axial least stable positions. There is a severe crowding among the atoms in axial positions on the same side of the ring. The boat conformation is quite flexible. The chair conformation is considered to be the lowest energy conformation because it lacks both angle strain and torsional strainThis means that the chair conformation is the structure. The chair conformation has alternating axial up axial down so once you have that single axial substituent move on to.
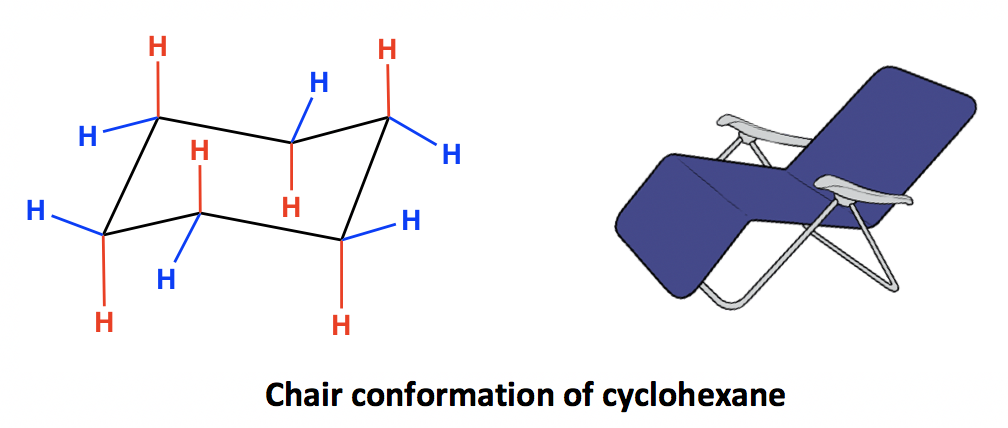 Source: kpu.pressbooks.pub
Source: kpu.pressbooks.pub
4 3 Conformation Analysis Of Cyclohexane Organic Chemistry Using the numbering scheme in the original structure shown in step 1 and the numbering scheme in the chair conformation templates drawn in step 3 fill in the substituents on the chair conformations. Answer this question WITHOUT drawing a chair conformationring flip. Number the ring and draw any chair conformation of the compound. Drawing a Sugars Chair Conformation from a Haworth Structure or Fischer Projection If you have either the Haworth structure or the Fischer projection of the sugar drawing the chair conformation of the sugar is easy as long as you remember the orientations of the axial and equatorial bonds on each carbon atom of the chair conformation. The stability is a consequence of no angle strain owing to the almost tetrahedral C-C-C bond angles and no torsional strain due to the perfectly staggered. Using the numbering scheme in the original structure shown in step 1 and the numbering scheme in the chair conformation templates drawn in step 3 fill in the substituents on the chair conformations.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Cyclohexane Chair Conformation Stability Which One Is Lower Energy In each of the two boxes below draw in a bond to one methyl CH3 group in the appropriate position. The definition of chair conformation is the lowest energy conformation for cyclohexane in which all bond angles are fairly close to 1095 and all hydrogen atoms are staggered. Use the guidelines below place substituents in the proper axialequatorial orientation. Using the numbering scheme in the original structure shown in step 1 and the numbering scheme in the chair conformation templates drawn in step 3 fill in the substituents on the chair conformations. In each of the two boxes below draw in a bond to one methyl CH3 group in the appropriate position. The chair conformation has alternating axial up axial down so once you have that single axial substituent move on to.
 Source: study.com
Source: study.com
Draw The Chair Conformations Of Each Of The Following Molecules Indicating Which One Should Be More Stable And Briefly Justify Your Reasoning Study Com The chair structure consists of a six-membered ring where every C-C bond exists in a staggered conformation. Although the hydrocarbon cyclohexane is typically drawn as if it were flat in reality the structure is not flat at all. By flexing to a new formthe twist conformation Fig5the boat conformation can relieve some of its torsional strain and at the same time reduce the flagpole interactions. This conformation of cyclohexane is called the chair conformation be-cause of its resemblance to a lawn chair. There is a severe crowding among the atoms in axial positions on the same side of the ring. Draw the second chair conformation ring-flip-check this post if not sure.
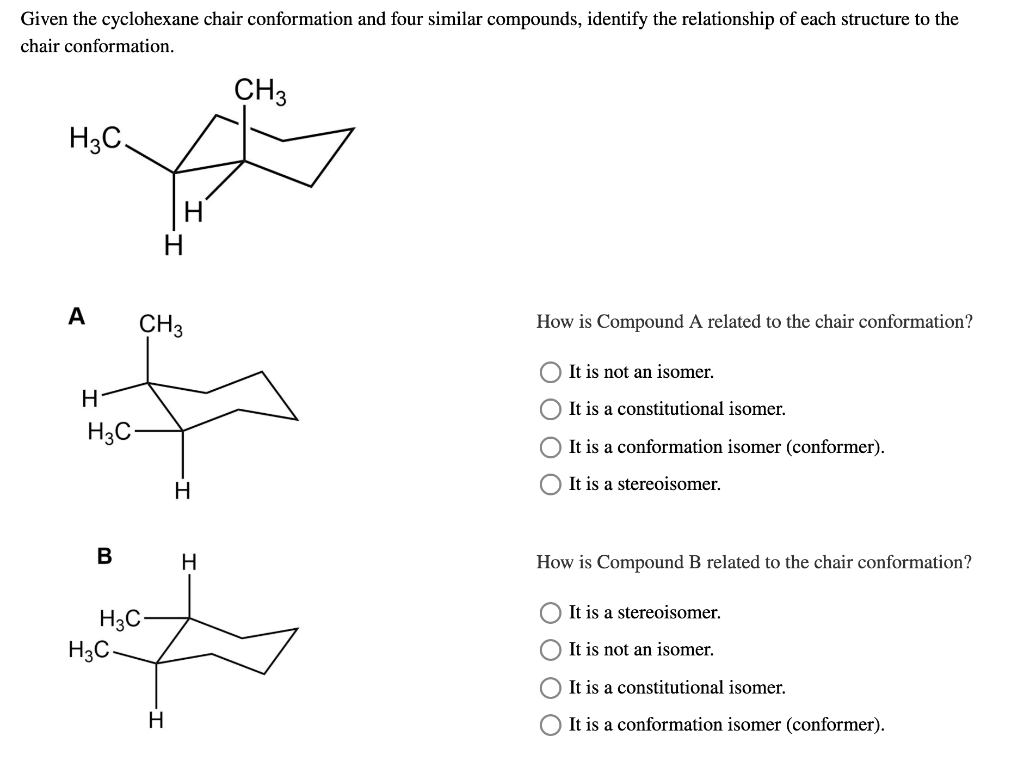 Source: chegg.com
Source: chegg.com
Solved Given The Cyclohexane Chair Conformation And Four Chegg Com This is your first axial substituent. The boat conformation is quite flexible. The chair structure of cyclohexane is considered to be the perfect conformation. Determine if the highlighted atom will appear in the axial or equatorial position in the more stable chair conformation. Number the ring and draw any chair conformation of the compound. Draw the second chair conformation ring-flip-check this post if not sure.
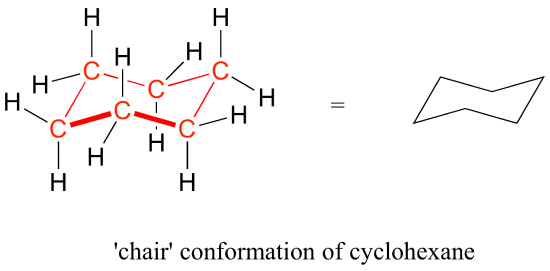 Source: chem.libretexts.org
Source: chem.libretexts.org
4 3 Conformations Of Cyclic Organic Molecules Chemistry Libretexts First we have to introduce the concept of an A-value which is simply the energy difference between the equatorial most stable and axial least stable positions. For each chair conformer add the energy of all the groups on axial position. The chair conformation is considered to be the lowest energy conformation because it lacks both angle strain and torsional strainThis means that the chair conformation is the structure. 2 pt CH3 Least stable chair Most stable chair 1 1 1 Ð18 kcalmolG. Explaining how A-Values are related to cyclohexane flip energy. The chair conformation being the most stable and predominant form of cyclohexane is non-planar with the headrest and footrest bonds lying above and below the planar seat bonds.

Chair Structure Course Hero Answer this question WITHOUT drawing a chair conformationring flip. Answer this question WITHOUT drawing a chair conformationring flip. In this post I want to go over the three most typical forms of the carbohydrates. First we have to introduce the concept of an A-value which is simply the energy difference between the equatorial most stable and axial least stable positions. The boat conformation is quite flexible. Although the hydrocarbon cyclohexane is typically drawn as if it were flat in reality the structure is not flat at all.
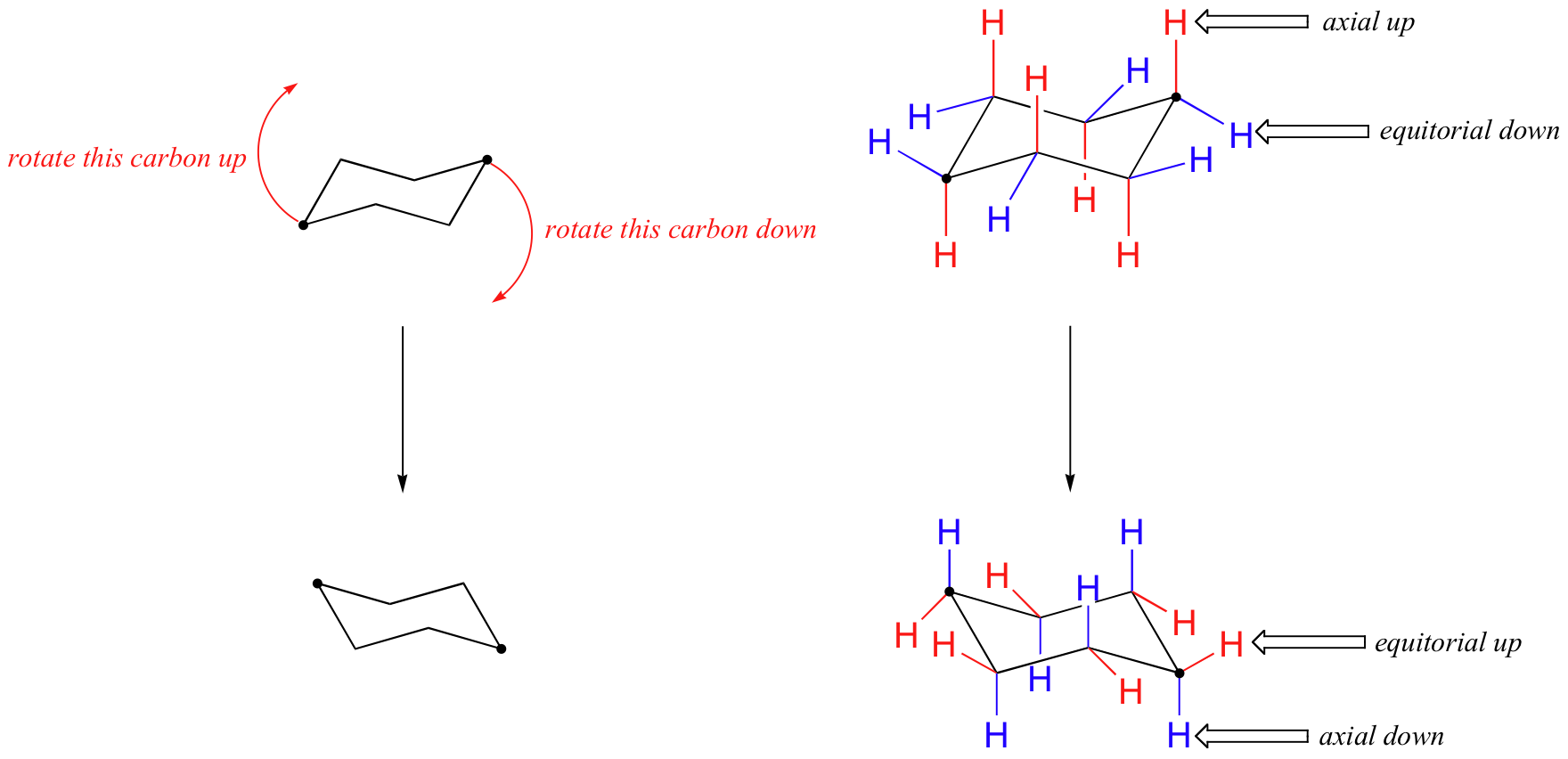 Source: chem.libretexts.org
Source: chem.libretexts.org
4 7 Cyclohexane Conformations Chemistry Libretexts The stability is a consequence of no angle strain owing to the almost tetrahedral C-C-C bond angles and no torsional strain due to the perfectly staggered. The chair conformation being the most stable and predominant form of cyclohexane is non-planar with the headrest and footrest bonds lying above and below the planar seat bonds. And now the stabilities. Although it is more stable the chair conformation is much more rigid than the boat conformation. Using the numbering scheme in the original structure shown in step 1 and the numbering scheme in the chair conformation templates drawn in step 3 fill in the substituents on the chair conformations. This conformation is called the chair because it looks sort of like a reclining lounge chair as shown here.
 Source: researchgate.net
Source: researchgate.net
Chemical Structures Of Benzene Top And Cyclohexane Bottom Benzene Download Scientific Diagram Explaining how A-Values are related to cyclohexane flip energy. There are twelve possible positions on a chair structure. The most stable conformation of cyclohexane is shown in Fig. In a chair conformation the bond angles for each carbon are about 109 degrees so the tetrahedrals are pretty close. The chair conformation being the most stable and predominant form of cyclohexane is non-planar with the headrest and footrest bonds lying above and below the planar seat bonds. In each of the two boxes below draw in a bond to one methyl CH3 group in the appropriate position.
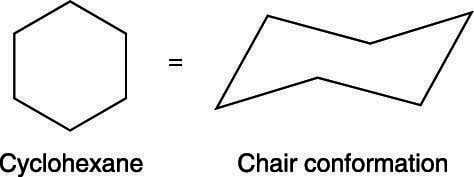 Source: dummies.com
Source: dummies.com
How To Draw The Chair Conformation Of Cyclohexane Dummies This organic chemistry video tutorial provides a basic introduction into drawing the chair conformation of cyclohexane and identifying the most stable confor. The chair conformation is considered to be the lowest energy conformation because it lacks both angle strain and torsional strainThis means that the chair conformation is the structure. The chair conformation being the most stable and predominant form of cyclohexane is non-planar with the headrest and footrest bonds lying above and below the planar seat bonds. Look at the axial-methylcyclohexane in chair conformation shown. 2 pt CH3 Least stable chair Most stable chair 1 1 1 Ð18 kcalmolG. Notice how the axial-methyl group is thrown closely together with the axial hydrogens in carbons 3 and 5.
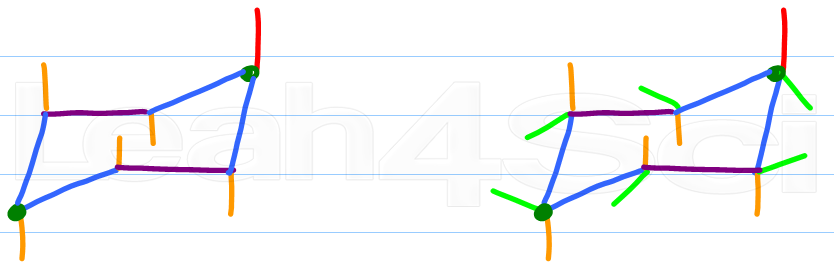 Source: leah4sci.com
Source: leah4sci.com
Drawing Chair Conformations And Ring Flips For Cyclohexane In Organic Chemistry Most of the time the structure exists in what is called the chair conformation. The most stable conformation of cyclohexane is shown in Fig. In each of the two boxes below draw in a bond to one methyl CH3 group in the appropriate position. And now the stabilities. Determine if the highlighted atom will appear in the axial or equatorial position in the more stable chair conformation. By flexing to a new formthe twist conformation Fig5the boat conformation can relieve some of its torsional strain and at the same time reduce the flagpole interactions.
 Source: researchgate.net
Source: researchgate.net
Cyclohexane Conformations A Top Chair Conformation B Middle Download Scientific Diagram Although the hydrocarbon cyclohexane is typically drawn as if it were flat in reality the structure is not flat at all. This organic chemistry video tutorial provides a basic introduction into drawing the chair conformation of cyclohexane and identifying the most stable confor. Although it is more stable the chair conformation is much more rigid than the boat conformation. Use the guidelines below place substituents in the proper axialequatorial orientation. In this post I want to go over the three most typical forms of the carbohydrates. The Stereochemistry of the Anomeric Carbon the α-form or the β-form Converting Haworth to Chair.
 Source: en.wikipedia.org
Source: en.wikipedia.org
File Cycloheptane Chair Conformation Svg Wikipedia The boat conformation is quite flexible. Determine if the highlighted atom will appear in the axial or equatorial position in the more stable chair conformation. Answer this question WITHOUT drawing a chair conformationring flip. The most stable conformation of cyclohexane is shown in Fig. This conformation is called the chair because it looks sort of like a reclining lounge chair as shown here. The Stereochemistry of the Anomeric Carbon the α-form or the β-form Converting Haworth to Chair.
Please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save or be able to bookmark this blog page in this website. Thank you …










