Wikipedia chair conformation DF Bocian HM Pickett TC Rounds HL Strauss 1975. Both the equatorial and axial positions can be either up or down.
Wikipedia Chair Conformation, SVG development The source code of this SVG is valid. The configuration of all stereotopic atoms and about the constitution of the molecule. If the bond angles were significantly distorted from tetrahedral we would expect to see a greater.
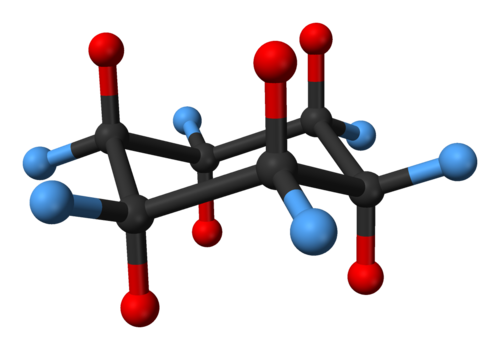 Cyclohexane Conformation Wikiwand From wikiwand.com
Cyclohexane Conformation Wikiwand From wikiwand.com
Another Article :
First we have to introduce the concept of an A-value which is simply the energy difference between the equatorial most stable and axial least stable positions. The Chair Conformation The stability data in Table 71 require that the bond angles in cyclohexane must be essentially the same as the bond angles in an alkanevery close to the ideal 1095 tetrahedral angle. Its not angle strain - all the bonding angles in either the chair or the boat are 109. The gauche conformation on the right is a conformer while the eclipsed conformation on the left is a transition state between. Chair conformation stereochemistry axatorial equial.
The conformation thus also contains information about the stereochemistry ie.
The conformation of an organic molecule describes the spatial arrangement of its rotatable bonds on the carbon atoms. Chair left and boat right conformation of a general cyclohexane structure a axial substituent e equatorial substituent Päiväys 26. Both the equatorial and axial positions can be either up or down. This first conformation is called the chair conformation. This is true for 1R-33-dichlorocyclohexanol.
 Source: wikiwand.com
Source: wikiwand.com
Ring Flip Wikiwand The configuration of all stereotopic atoms and about the constitution of the molecule. A cyclohexane molecule in chair conformation. As we can see from Scheme 13 there is a steric clash in the boat conformation. DF Bocian HM Pickett TC Rounds HL Strauss 1975. First we have to introduce the concept of an A-value which is simply the energy difference between the equatorial most stable and axial least stable positions. The conformation thus also contains information about the stereochemistry ie.
 Source: in.pinterest.com
Source: in.pinterest.com
Stereoisomerism Organic Chemistry Mechanisms Organic Chemistry Teaching Chemistry The Chair Conformation The stability data in Table 71 require that the bond angles in cyclohexane must be essentially the same as the bond angles in an alkanevery close to the ideal 1095 tetrahedral angle. This is true for 1R-33-dichlorocyclohexanol. DF Bocian HM Pickett TC Rounds HL Strauss 1975. Cyclohexane conformations including with chair and boat conformers among others. The term Ring flip is used to describe the conversion of one chair conformation into the other. Chair conformation plural chair conformations chemistry the most stable chemical conformation of a six-membered single bonded carbon ring such as cyclohexane.
 Source: pinterest.com
Source: pinterest.com
Cyclohexane And Chair Flipping Intermediate Boat Conformation Organic Chemistry Video Organic Chemistry Chemistry Online School Explaining how A-Values are related to cyclohexane flip energy. Carbohydrate conformation which includes. First we have to introduce the concept of an A-value which is simply the energy difference between the equatorial most stable and axial least stable positions. Use of the information documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice and subject to other binding limitations provided for under applicable law the information documents and data made available on the ECHA website may be reproduced distributed andor used totally or in part for non-commercial purposes provided that ECHA is. This is an elegant demonstration of how NMR helps to visualize the pyranose cycle chair conformation. In a chair conformation with a few exceptions only axial substituents will give rise to steric strain energy.
 Source: wikiwand.com
Source: wikiwand.com
Cyclohexane Conformation Wikiwand Use of the information documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice and subject to other binding limitations provided for under applicable law the information documents and data made available on the ECHA website may be reproduced distributed andor used totally or in part for non-commercial purposes provided that ECHA is. In a chair conformation with a few exceptions only axial substituents will give rise to steric strain energy. A six-membered ring conformation in which atoms 2 3 5 and 6 lie in the same plane atom 1 lies above the plane and atom 4 lies below the plane. Its not angle strain - all the bonding angles in either the chair or the boat are 109. Equilibrium usually favors the conformation with the equatorial substituent because 13-diaxial gauche interactions are gone. Both the equatorial and axial positions can be either up or down.

File Cyclooctane Boat Chair Conformation Svg Wikipedia This is true for 1R-33-dichlorocyclohexanol. Public domain Public domain false false. A cyclohexane molecule in chair conformation. Source for name and structure. The conformation thus also contains information about the stereochemistry ie. The conformation of an organic molecule describes the spatial arrangement of its rotatable bonds on the carbon atoms.
 Source: pinterest.com
Source: pinterest.com
Akbash Dog 2 Years White White Eyes Akbash Dog Dog Breeds Dog Friends In a chair conformation with a few exceptions only axial substituents will give rise to steric strain energy. Source for name and structure. This is an elegant demonstration of how NMR helps to visualize the pyranose cycle chair conformation. Is CIS axial or equatorial. The Journal of Physical Chemistry. Cyclooctane twist chair-chair conformation.
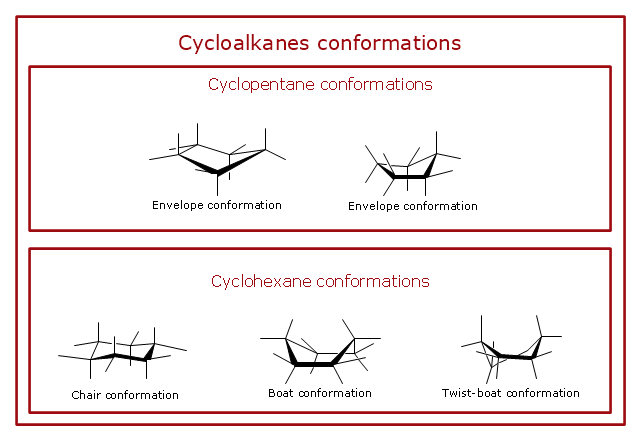 Source: conceptdraw.com
Source: conceptdraw.com
Cycloalkanes Conformations Cyclopentane Half Chair The Chair Conformation The stability data in Table 71 require that the bond angles in cyclohexane must be essentially the same as the bond angles in an alkanevery close to the ideal 1095 tetrahedral angle. Usually chair conformation is the most stable conformation and at room temperature about 9999 of cyclohexane in a mixture of different conformation exists in this conformation. PW Pakes TC Rounds HL Strauss 1981. This first conformation is called the chair conformation. The term Ring flip is used to describe the conversion of one chair conformation into the other. In a chair conformation with a few exceptions only axial substituents will give rise to steric strain energy.
 Source: commons.wikimedia.org
Source: commons.wikimedia.org
File Cyclohexane Conformations And Energies With Hydrogens Svg Wikimedia Commons This first conformation is called the chair conformation. Cis and trans are determined by if the molecules are up or down in relation to the ring. Explaining how A-Values are related to cyclohexane flip energy. Is CIS axial or equatorial. Cyclohexane conformations including with chair and boat conformers among others. In organic chemistry cyclohexane conformations are any of several three-dimensional shapes adopted by molecules of cyclohexaneBecause many compounds feature structurally similar six-membered rings the structure.
 Source: pinterest.com
Source: pinterest.com
The Beckmann Rearrangement Organic Chemistry Chemistry Basic Facts Calculating Flip Energy. Public domain Public domain false false. If the bond angles were significantly distorted from tetrahedral we would expect to see a greater. A six-membered ring conformation in which atoms 2 3 5 and 6 lie in the same plane atom 1 lies above the plane and atom 4 lies below the plane. Chair conformation plural chair conformations chemistry the most stable chemical conformation of a six-membered single bonded carbon ring such as cyclohexane. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators.
 Source: fi.m.wikipedia.org
Source: fi.m.wikipedia.org
Tiedosto Chair Boat Conformation General Svg Wikipedia Source for name and structure. Cyclooctane twist chair-chair conformationsvg. They completely describe the three-dimensional spatial coordinates of all atoms in the molecule. Both the equatorial and axial positions can be either up or down. A cyclohexane molecule in chair conformation. Moreover the symmetry of chair conformation is D 3d while boat symmetry has the symmetry C 2v.
 Source: pinterest.com
Source: pinterest.com
The Relative Rate Of E2 Reaction For Substituted Cyclohexanes Study Chemistry Organic Chemistry Chemistry Classroom This structural formula was created with ChemDraw. The configuration of all stereotopic atoms and about the constitution of the molecule. Use of the information documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice and subject to other binding limitations provided for under applicable law the information documents and data made available on the ECHA website may be reproduced distributed andor used totally or in part for non-commercial purposes provided that ECHA is. Explaining how A-Values are related to cyclohexane flip energy. Chair conformation of cyclohexane. First we have to introduce the concept of an A-value which is simply the energy difference between the equatorial most stable and axial least stable positions.
 Source: pinterest.com
Source: pinterest.com
Cyclohexane Chair Conformation Stability Which One Is Lower Solved On A Cyclohexane Ring An Axial Carboxyl Group Has Boat Confor Methyl Group Axial Chemistry This is true for 1R-33-dichlorocyclohexanol. PW Pakes TC Rounds HL Strauss 1981. The configuration of all stereotopic atoms and about the constitution of the molecule. This structural formula was created with ChemDraw. The step size of 05 fs with 10 steps. First we have to introduce the concept of an A-value which is simply the energy difference between the equatorial most stable and axial least stable positions.
 Source: pinterest.com
Source: pinterest.com
Favorskii Rearrangement Chimie Paysage Fantastique In each of the conformations drawn in step 4 circle the axial substituents other than hydrogen. Public domain Public domain false false. Its not angle strain - all the bonding angles in either the chair or the boat are 109. Chair left and boat right conformation of a general cyclohexane structure a axial substituent e equatorial substituent Päiväys 26. Is CIS axial or equatorial. Equilibrium usually favors the conformation with the equatorial substituent because 13-diaxial gauche interactions are gone.
 Source: pinterest.com
Source: pinterest.com
Spectrometer Spectrometers Chemistry Help Teaching Chemistry In organic chemistry cyclohexane conformations are any of several three-dimensional shapes adopted by molecules of cyclohexaneBecause many compounds feature structurally similar six-membered rings the structure. SVG development The source code of this SVG is valid. Hydrogen atoms in axial positions are shown in red while those in equatorial positions are in blue. Two possible chair conformations can be drawn one where the substituent is axial and one where it is equatorial. Source for name and structure. In a chair conformation with a few exceptions only axial substituents will give rise to steric strain energy.
 Source: wikiwand.com
Source: wikiwand.com
Cyclohexane Conformation Wikiwand Calculating Flip Energy. Hydrogen atoms in axial positions are shown in red while those in equatorial positions are in blue. This structural formula was created with ChemDraw. Cyclooctane twist chair-chair conformation. Source for name and structure. The configuration of all stereotopic atoms and about the constitution of the molecule.
 Source: pinterest.com
Source: pinterest.com
Starch And Cellulose Cellulose Starch Chemistry To be cis they must be either both up or both down regardless of if they. Public domain Public domain false false. Moreover the symmetry of chair conformation is D 3d while boat symmetry has the symmetry C 2v. The conformation thus also contains information about the stereochemistry ie. The Journal of Physical Chemistry. Chair conformation of cyclohexane.
 Source: in.pinterest.com
Source: in.pinterest.com
El Ununseptio El Elemento 117 De La Tabla Periodica Dibujo20140508 Ununseptium Decay Chain Aps Physics Prl Chemistry Labs Chemistry Science SVG development The source code of this SVG is valid. Usually chair conformation is the most stable conformation and at room temperature about 9999 of cyclohexane in a mixture of different conformation exists in this conformation. There is another kind of strain. A cyclohexane molecule in chair conformation. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. The conformation thus also contains information about the stereochemistry ie.
 Source: fr.pinterest.com
Source: fr.pinterest.com
Restaurant Kong Hans Kaelder Copenhagen Dk Copenhagen Restaurants Copenhagen Fine Restaurant First we have to introduce the concept of an A-value which is simply the energy difference between the equatorial most stable and axial least stable positions. The conformation of an organic molecule describes the spatial arrangement of its rotatable bonds on the carbon atoms. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. Carbohydrate conformation which includes. Calculating Flip Energy. DF Bocian HM Pickett TC Rounds HL Strauss 1975.
Please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark or be able to bookmark this blog page in this website. Thank you …










